Download
Details
Criteria
- People who meet the following criteria must meet the Juntendo University Hospital Immunization Requirements:
- Will have physical or face to face interaction with a patient.
- Will have contact with potentially contaminated items including, but not limited to, blood and/or body fluids.
General Information
- The Juntendo University Hospital Immunization Requirements are based on the standards set by the Japanese Society for Infection Prevention and Control.
- All supporting documents must be submitted in either English or Japanese.
- All immunization requirements must be certified by a healthcare organization.
- All vaccinations and titer report dates must list a day, month, and year.
- Units: milli-international units / milliliters (mIU/ml) or Enzyme Immunoassay (EIA).
- Other units may be acceptable. Contact Juntendo University International Center (juic@juntendo.ac.jp) for details.
- Applicants who have not met the immunization requirements at the time of their application must complete the Juntendo University Hospital Immunization Requirements Pledge.
Proof
- One healthcare provider does not need to issue all of the immunization requirements. For example you could submit:
- one immunization requirements form issued by one healthcare provider with the measles, mumps, and rubella and varicella requirements complete and
- another immunization requirements form issued by another healthcare provider with the hepatitis B, tuberculosis, and influenza requirement complete.
- If a healthcare provider issues immunization requirements in a language other than English or Japanese, ask the healthcare provider to fill in the results on the Juntendo University Immunization Requirements form.
- If the immunization requirements contain medical information not related to the Juntendo University immunization requirements, be sure to highlight the information relevant to the Juntendo University immunization requirements.
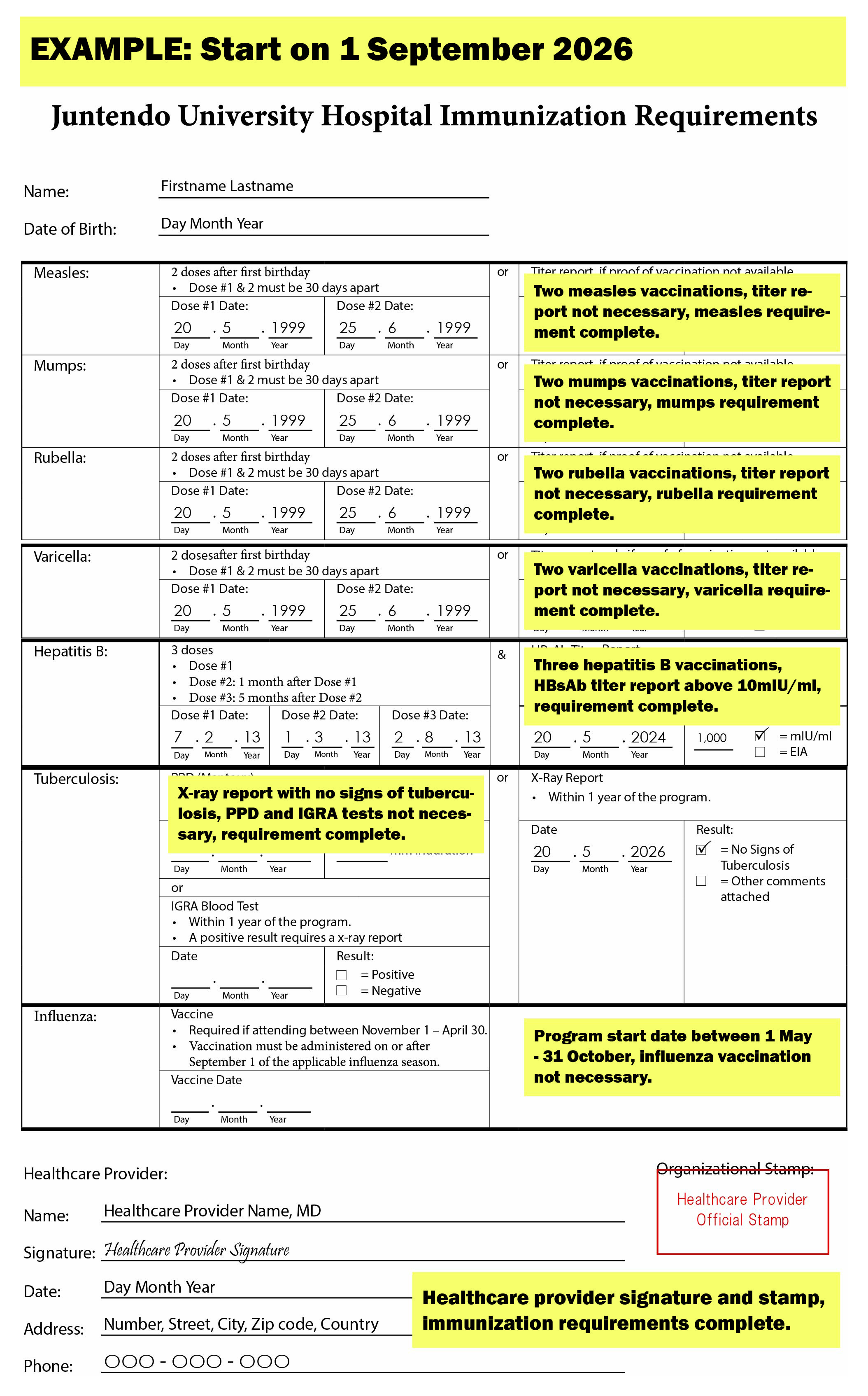
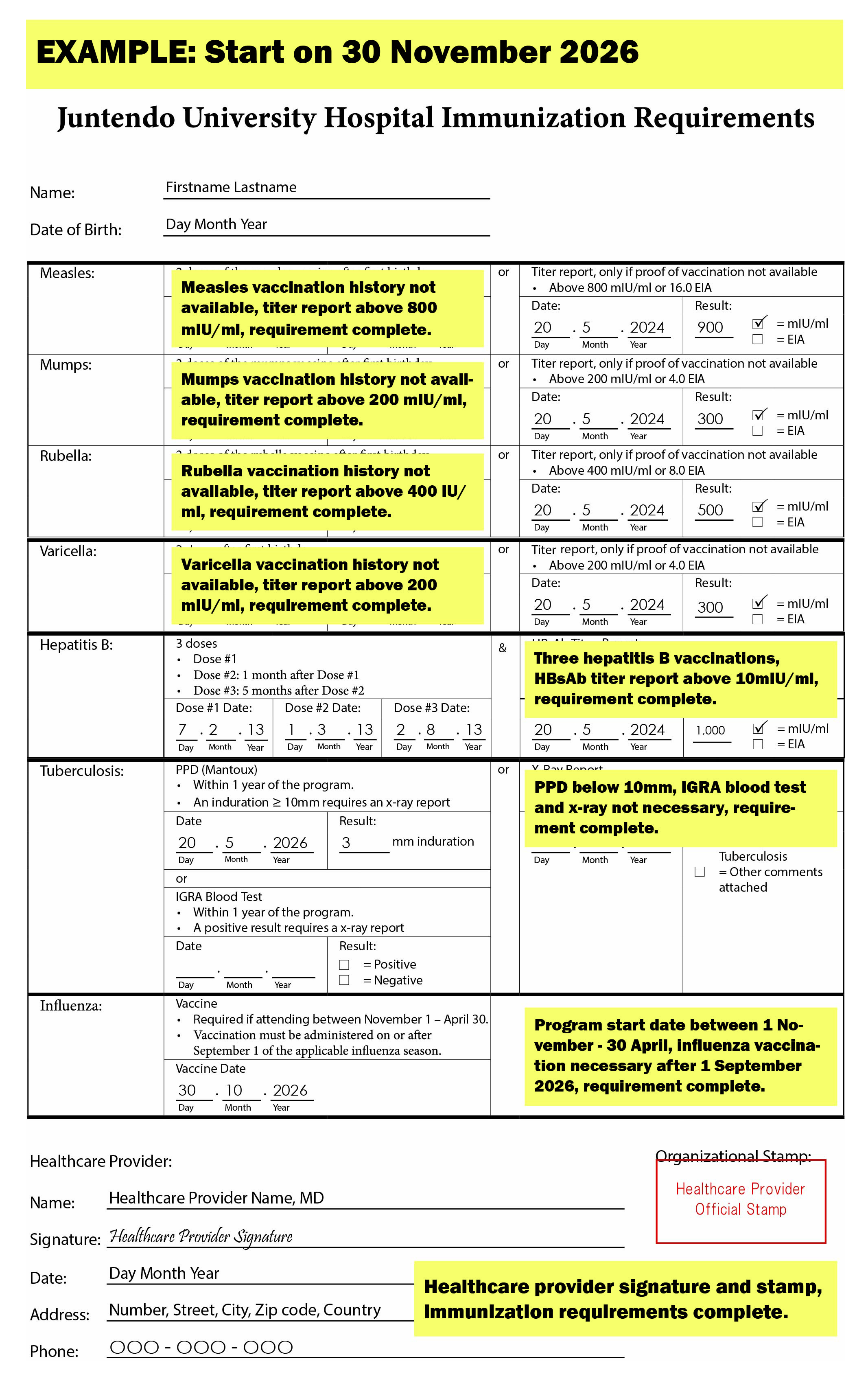
Measles, Mumps, Rubella, and Varicella Requirements
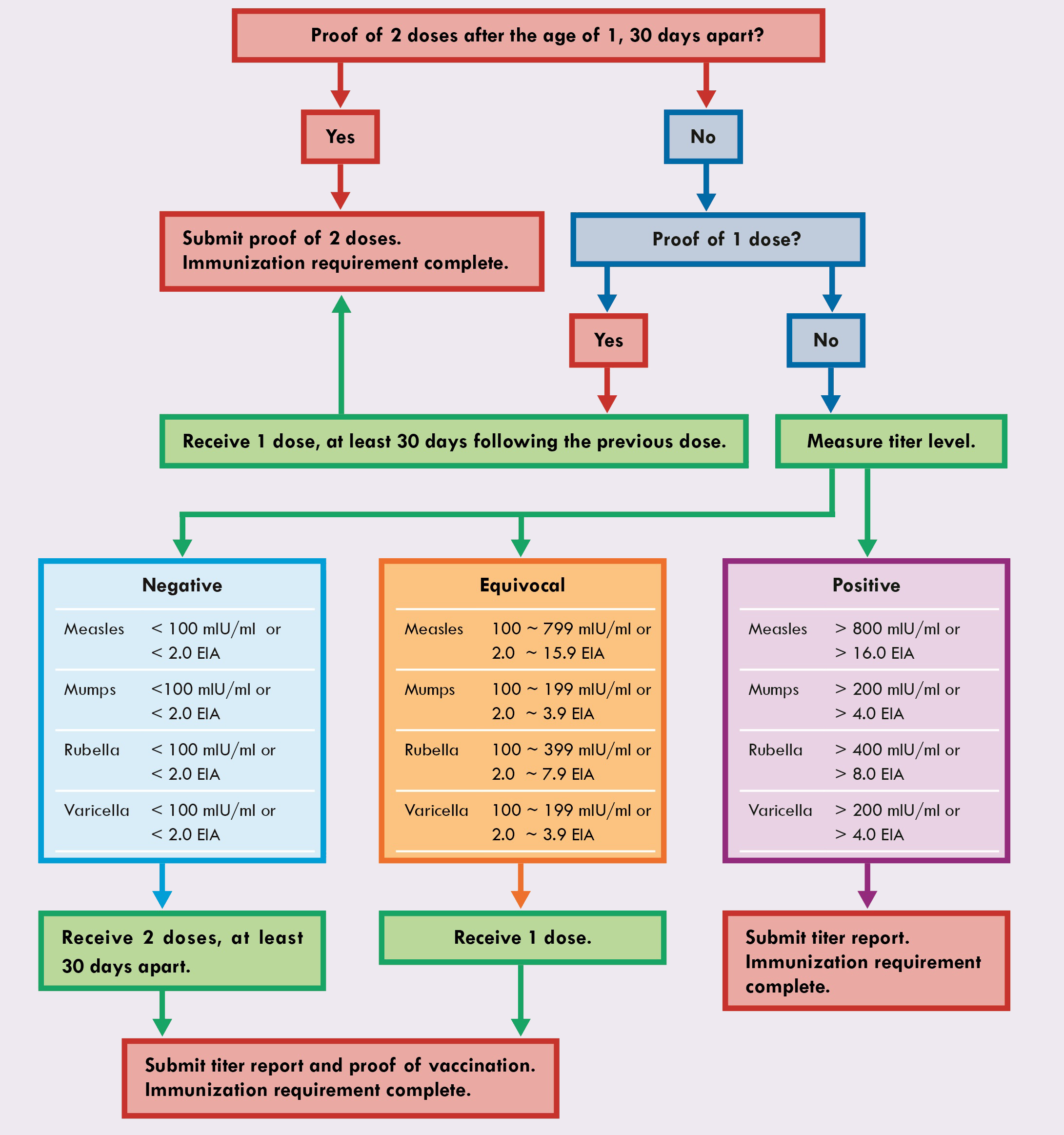
- Submit proof of two doses of the vaccine at least 30 days apart, received after the age of one.
- If you have proof of one dose received after the age of one, receive an additional dose, at least 30 days after the first dose and submit proof of the two doses.
- If you do not have proof of two doses, you may get a serology test and submit the titer report and proof of vaccination if necessary. The tables below indicate the reference intervals.
- A negative titer report requires two doses at least 30 days apart following the serology test.
- An equivocal titer report requires a dose following the serology test.
- A positive titer report does not require a dose following the serology test.
- A titer report must list an exact level.
- Detected or positive titer reports without an exact level do not meet the requirements. To meet the requirements submit an index that determines how the titer report classified the detected or positive result.
- A history of chicken pox does not meet the varicella requirement.
Hepatitis B Requirements
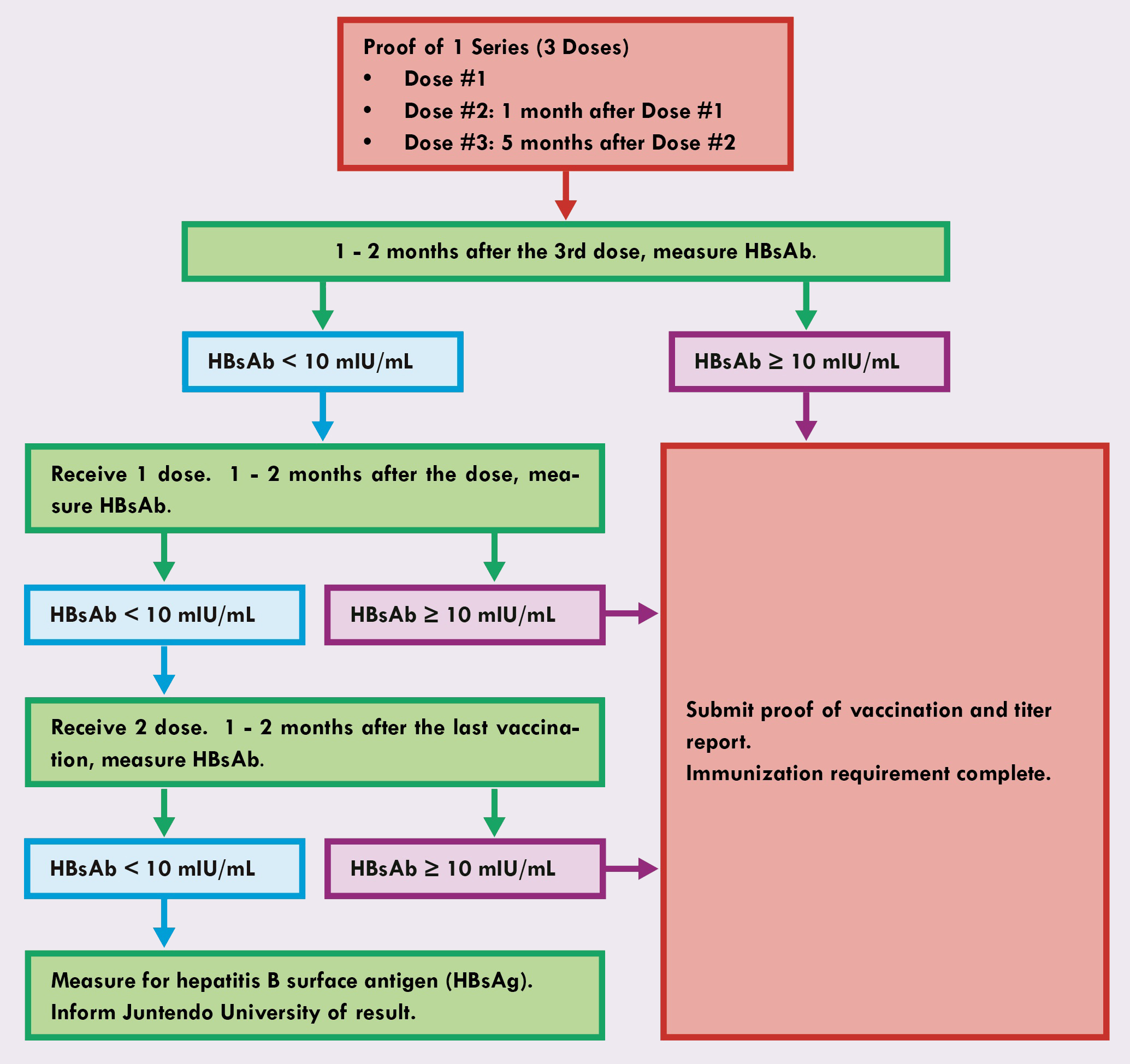
- Submit proof of one vaccination series (3 doses) of the hepatitis B vaccine and a hepatitis B surface antibody (HBsAb) titer report greater than or equal to 10 mIU/ml, 1 - 2 months after the last dose.
- The HBsAb titer report must list an exact level.
- A positive titer report without an exact level does not meet the requirements. To meet the requirements submit an index that determines how the titer report classified the positive result.
- If a record of previous hepatitis B vaccination is not available, but proof of an HBsAb test greater than or equal to 10 mIU/ml is, submit the proof of the test result, the HB requirement is complete.
- After three doses of the hepatitis B vaccine, if your HBsAb titer report is below 10 mIU/ml receive an additional dose of the HB vaccine. 1 - 2 months after the last dose, undergo an HBsAb test. If the titer report is above 10 mIU/ml, submit proof of vaccination and the titer report to meet the requirements.
- After four doses of the hepatitis B vaccine, if your HBsAb titer report remains below 10 mIU/ml receive an additional two doses. 1 - 2 months after the last dose, undergo an HBsAb test. If the titer report is above 10 mIU/ml, submit proof of vaccination and the titer report to meet the requirements.
- After two vaccination series (six doses) of the hepatitis B vaccine if your HBsAb titer report is below 10 mIU/ml take a hepatitis B surface antigen (HBsAg) test. Inform Juntendo University International Center (juic@juntendo.ac.jp) of the HBsAg results to determine how to proceed.
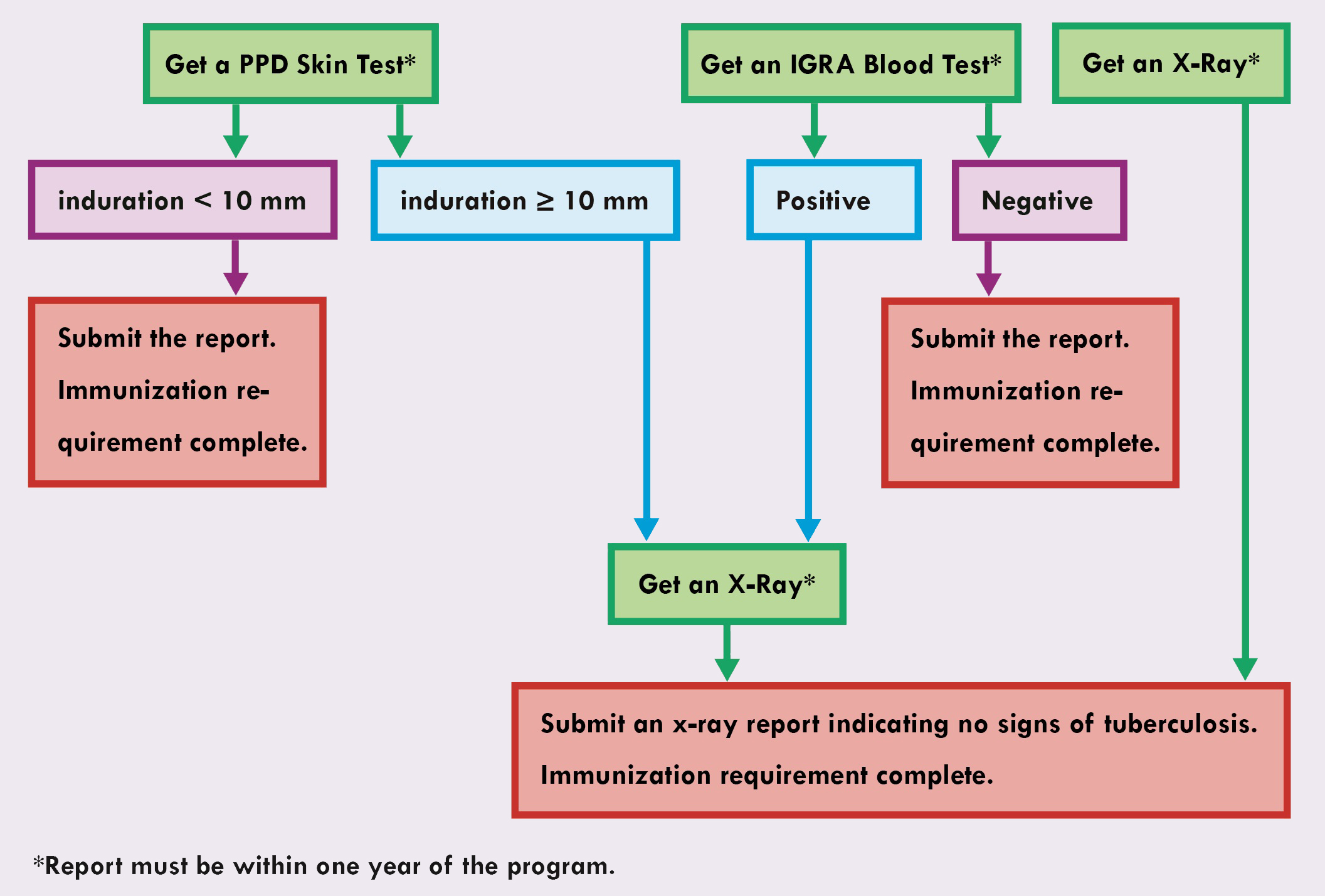
Tuberculosis Requirement
- Submit one of the following reports dated within one year of your program start date:
- Purified Protein Derivative (PPD) skin test with an induration < 10 mm
- Interferon-Gamma Release Assays (IGRA) blood test with a negative result
- X-ray report with no signs of TB
- If you have a PPD test result with an induration greater than or equal to 10mm, submit an x-ray report indicating no signs of tuberculosis to meet the requirement.
- If you have a positive IGRA test result, submit an x-ray report indicating no signs of tuberculosis to meet the requirement.
- Proof a Bacille Calmette-Guerin (BCG) vaccination does not meet the tuberculosis requirement.
Influenza Requirement
- For participants attending between November 1 and April 30, the influenza vaccine must have been administered on or after September 1 of the applicable influenza season (i.e., the most recent flu season prior to attendance), regardless of geographic region, country of residence, or vaccination location.
- For participants attending between May 1 and September 30, proof of influenza vaccination is not required.
- For participants whose program begins before November 1 and extends into the influenza season (November 1 – April 30), the influenza vaccination may be administered in Japan during their program.
Immunization Requirement Completed Form Examples