Jul. 19, Wed, 2023
Topics
>RESEARCH
New Study Throws Light on Mechanisms Underlying Helicobacter pylori-Induced Gastric Cancer
Researchers from Japan show how oncoprotein CagA from Helicobacter pylori disrupts Wnt/PCP signaling and promotes gastric carcinogenesis
Helicobacter pyloricagA+ strains cause gastric inflammation, ulcers, and cancer. Previous reports have shown that the cagA gene-encoded protein “CagA” plays a crucial role in gastric carcinogenesis. However, the underlying mechanisms remain to be fully elucidated. Researchers from Japan used animal models and laboratory-cultured human gastric epithelial cells to demonstrate that CagA works by disrupting the Wnt/Planar Cell Polarity (PCP) signaling pathway.
The Mechanism UnderlyingHelicobacter pyloriOncoprotein CagA Action and Development of Gastric Cancer
The Mechanism UnderlyingHelicobacter pyloriOncoprotein CagA Action and Development of Gastric Cancer
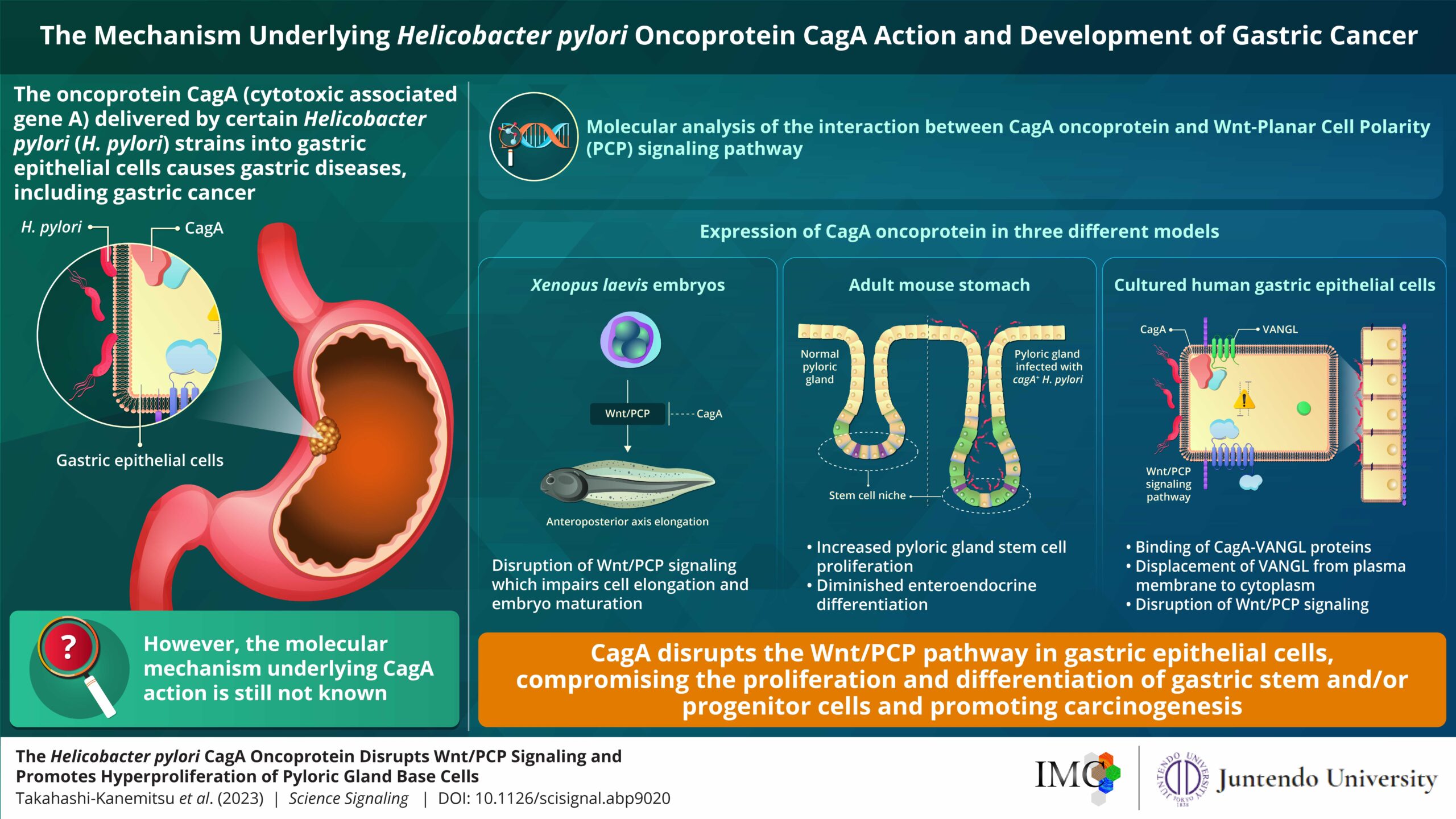
The disruption of Wnt/PCP signaling by the oncoprotein CagA seems to promote gastric carcinogenesis.
License type:Original Content
Usage restrictions: You are free to share and adapt the Infographic material but attribution is required, with a link to the news source.
License type:Original Content
Usage restrictions: You are free to share and adapt the Infographic material but attribution is required, with a link to the news source.
Helicobacter pylori (H. pylori) infections are commonly associated with abdominal pain, bloating, and acidity. Clinical evidence suggests that infection with H. pylori cagA+ strains dramatically increases the risk of developing gastric cancer. A specialized protein delivered by H. pylori to the host, oncoprotein “CagA,” has been shown to interact with multiple host proteins and promote gastric carcinogenesis (transformation of normal cells to cancer cells). However, the underlying mechanisms associated with its biochemical activity have not been fully determined yet.
A new study published in Science Signaling on 18 July 2023 shares insights into the additional mechanism of oncogenic CagA action. “CagA interacts with multiple host proteins within the gastric epithelial cells, thereby inducing pathways associated with oncogenesis and promoting gastric carcinogenesis. We were curious to find out which pathways were involved in this process,’ says Dr. Atsushi Takahashi-Kanemitsu, lead author of the study and Assistant Professor, Department of Biochemistry & Systems Biomedicine, Juntendo University, as he states the motivation behind pursuing this study.
In order to carry out their study, the researchers expressed oncoprotein CagA in three different models—embryos of Xenopus laevis (lab frog), adult mouse stomach, and cultured human gastric epithelial cells—and tried to understand its effect on the host cells and pathways.
The team noted that the expression of the CagA oncoprotein in X. laevis embryos led to impairment of convergent extension movements—cell movements observed during embryonic development that are involved in shaping or elongating organismal tissues and organs. This impairment further interfered with subsequent key embryonic development processes, including body axis formation.
Similarly, the team performed an experiment using adult mice. They generated genetically modified (transgenic) mice that specifically express the CagA oncoprotein in the stomach epithelial cells in response to tamoxifen treatment.
The researchers observed that CagA expression in the stomach of adult mice caused an increase in the depth of pyloric glands—secretory glands that facilitate digestion/stomach function—and also triggered abnormal/excessive cell multiplication, which is a phenomenon remarkably observed in various types of cancers. This also led to the displacement of the proteins “VANGL1/2”—members of the Van Gogh-like (VANGL) protein family, which play key roles in various biological processes—from the plasma membrane to the cytoplasm. CagA expression also resulted in fewer differentiated enteroendocrine cells, which are specialized cells in the gastrointestinal tract that aid in digestion.
Finally, the team expressed the CagA oncoprotein in cultured human gastric epithelial cells. The experiments clearly demonstrated that a small region of the CagA oncoprotein was interacting with amino acid residues from the proteins VANGL1/2, thus leading to its displacement (a phenomenon also observed in the mouse model) and resulting in disruption of the Wnt/PCP pathway—a key biological ‘relay’ that affects organismal development.
Corresponding author Masanori Hatakeyama, Laboratory Head, Institute of Microbial Chemistry, Microbial Chemistry Research Foundation, says, “Perturbation of Wnt/PCP signaling by the H. pylori CagA-VANGL interaction induces hyperplastic changes, along with impaired cell differentiation in gastric pyloric glands. This, in conjunction with other oncogenic CagA actions, may contribute to the development of gastric cancer.”
In summary, the researchers conclude that through this study, they were able to elucidate the molecular mechanisms involved in gastric carcinogenesis induced by H. pylori, gain insights into the role of the Wnt/PCP pathway in carcinogenesis, and propose it as a potential target for clinical interventions against H. pylori cagA+ infections.
This research project was conducted by Atsushi Takahashi-Kanemitsu (Juntendo University), Masanori Hatakeyama (Institute of Microbial Chemistry, Hokkaido University and The University of Tokyo), and Mengxue Lu (The University of Tokyo) in collaboration with Christopher T. Knight (The University of Tokyo), Takayoshi Yamamoto (The University of Tokyo), Takuo Hayashi (Juntendo University), Yusuke Mii (National Institute for Basic Biology, ExCELLS, and JST), Masanori Taira (Chuo University), Etsuo A. Susaki (Juntendo University), Nick Barker (A*STAR Singapore, Kanazawa University, and National University of Singapore), Takuya Ooki (Institute of Microbial Chemistry), Ippei Kikuchi (Institute of Microbial Chemistry), and Akira Kikuchi (Osaka University).
In order to carry out their study, the researchers expressed oncoprotein CagA in three different models—embryos of Xenopus laevis (lab frog), adult mouse stomach, and cultured human gastric epithelial cells—and tried to understand its effect on the host cells and pathways.
The team noted that the expression of the CagA oncoprotein in X. laevis embryos led to impairment of convergent extension movements—cell movements observed during embryonic development that are involved in shaping or elongating organismal tissues and organs. This impairment further interfered with subsequent key embryonic development processes, including body axis formation.
Similarly, the team performed an experiment using adult mice. They generated genetically modified (transgenic) mice that specifically express the CagA oncoprotein in the stomach epithelial cells in response to tamoxifen treatment.
The researchers observed that CagA expression in the stomach of adult mice caused an increase in the depth of pyloric glands—secretory glands that facilitate digestion/stomach function—and also triggered abnormal/excessive cell multiplication, which is a phenomenon remarkably observed in various types of cancers. This also led to the displacement of the proteins “VANGL1/2”—members of the Van Gogh-like (VANGL) protein family, which play key roles in various biological processes—from the plasma membrane to the cytoplasm. CagA expression also resulted in fewer differentiated enteroendocrine cells, which are specialized cells in the gastrointestinal tract that aid in digestion.
Finally, the team expressed the CagA oncoprotein in cultured human gastric epithelial cells. The experiments clearly demonstrated that a small region of the CagA oncoprotein was interacting with amino acid residues from the proteins VANGL1/2, thus leading to its displacement (a phenomenon also observed in the mouse model) and resulting in disruption of the Wnt/PCP pathway—a key biological ‘relay’ that affects organismal development.
Corresponding author Masanori Hatakeyama, Laboratory Head, Institute of Microbial Chemistry, Microbial Chemistry Research Foundation, says, “Perturbation of Wnt/PCP signaling by the H. pylori CagA-VANGL interaction induces hyperplastic changes, along with impaired cell differentiation in gastric pyloric glands. This, in conjunction with other oncogenic CagA actions, may contribute to the development of gastric cancer.”
In summary, the researchers conclude that through this study, they were able to elucidate the molecular mechanisms involved in gastric carcinogenesis induced by H. pylori, gain insights into the role of the Wnt/PCP pathway in carcinogenesis, and propose it as a potential target for clinical interventions against H. pylori cagA+ infections.
This research project was conducted by Atsushi Takahashi-Kanemitsu (Juntendo University), Masanori Hatakeyama (Institute of Microbial Chemistry, Hokkaido University and The University of Tokyo), and Mengxue Lu (The University of Tokyo) in collaboration with Christopher T. Knight (The University of Tokyo), Takayoshi Yamamoto (The University of Tokyo), Takuo Hayashi (Juntendo University), Yusuke Mii (National Institute for Basic Biology, ExCELLS, and JST), Masanori Taira (Chuo University), Etsuo A. Susaki (Juntendo University), Nick Barker (A*STAR Singapore, Kanazawa University, and National University of Singapore), Takuya Ooki (Institute of Microbial Chemistry), Ippei Kikuchi (Institute of Microbial Chemistry), and Akira Kikuchi (Osaka University).
This research is featured on the cover page of the July 18, 2023 issue of “Science Signaling”.
About Atsushi Takahashi-Kanemitsu, Ph.D from Juntendo University
Dr. Atsushi Takahashi-Kanemitsu works as an Assistant Professor at the Department of Biochemistry & Systems Biomedicine (Professor Etsuo A. Susaki’s Lab.), Juntendo University Graduate School of Medicine since 2022. He studied at the Division of Molecular Oncology, Institute for Genetic Medicine, Hokkaido University and received his Ph.D. in Chemistry from the Graduate School of Science, Hokkaido University in 2011. He then worked as a post-doctoral researcher and a research associate at Division of Microbiology, Graduate School of Medicine, The University of Tokyo (both of which are Professor Masanori Hatakeyama’s Labs) till 2021. He received the Research Fellowship for Young Scientists from Japan Society for the Promotion of Science (2007), and Annual Research Award from Japanese Association for Protein Phosphatase Research (2011). Google Scholar. Researchmap. ResearchGate. Twitter.
About Professor Masanori Hatakeyama from The University of Tokyo
Dr. Masanori Hatakeyama (Professor emeritus, The University of Tokyo) concurrently holds the position of Laboratory Head, Institute of Microbial Chemistry, Microbial Chemistry Research Foundation and Specially Appointed Professor at the Institute for Genetic Medicine, Hokkaido University. He received his MB (Bachelor of Medicine) from the School of Medicine, Hokkaido University in 1981 and passed the national examination for medical practitioners in 1981. He received his Ph.D degree from the Graduate School of Medicine, Hokkaido University in 1986. He then carried out his post-doctoral research at the Whitehead Institute, Massachusetts Institute of Technology (MIT), under the supervision of Professor Robert A. Weinberg. He is serving as Editor-in-Chief of Cancer Science, the official journal of the Japanese Cancer Association (JCA). He has been the recipient of many prizes and awards such as the Incitement Award by the Japanese Cancer Association (1991), JCA-Mauvernay Award (2006), Sagawa Special Award (2011), Medical Award of the Japan Medical Association (2014), Hideyo Noguchi Memorial Medical Prize (2016), Medal of Honor with Purple Ribbon (2019), Tomizo Yoshida Prize (2019), Princess Takamatsu Cancer Research Fund Prize (2022), and Takeda Prize for Medical Science (2022).
Dr. Atsushi Takahashi-Kanemitsu works as an Assistant Professor at the Department of Biochemistry & Systems Biomedicine (Professor Etsuo A. Susaki’s Lab.), Juntendo University Graduate School of Medicine since 2022. He studied at the Division of Molecular Oncology, Institute for Genetic Medicine, Hokkaido University and received his Ph.D. in Chemistry from the Graduate School of Science, Hokkaido University in 2011. He then worked as a post-doctoral researcher and a research associate at Division of Microbiology, Graduate School of Medicine, The University of Tokyo (both of which are Professor Masanori Hatakeyama’s Labs) till 2021. He received the Research Fellowship for Young Scientists from Japan Society for the Promotion of Science (2007), and Annual Research Award from Japanese Association for Protein Phosphatase Research (2011). Google Scholar. Researchmap. ResearchGate. Twitter.
About Professor Masanori Hatakeyama from The University of Tokyo
Dr. Masanori Hatakeyama (Professor emeritus, The University of Tokyo) concurrently holds the position of Laboratory Head, Institute of Microbial Chemistry, Microbial Chemistry Research Foundation and Specially Appointed Professor at the Institute for Genetic Medicine, Hokkaido University. He received his MB (Bachelor of Medicine) from the School of Medicine, Hokkaido University in 1981 and passed the national examination for medical practitioners in 1981. He received his Ph.D degree from the Graduate School of Medicine, Hokkaido University in 1986. He then carried out his post-doctoral research at the Whitehead Institute, Massachusetts Institute of Technology (MIT), under the supervision of Professor Robert A. Weinberg. He is serving as Editor-in-Chief of Cancer Science, the official journal of the Japanese Cancer Association (JCA). He has been the recipient of many prizes and awards such as the Incitement Award by the Japanese Cancer Association (1991), JCA-Mauvernay Award (2006), Sagawa Special Award (2011), Medical Award of the Japan Medical Association (2014), Hideyo Noguchi Memorial Medical Prize (2016), Medal of Honor with Purple Ribbon (2019), Tomizo Yoshida Prize (2019), Princess Takamatsu Cancer Research Fund Prize (2022), and Takeda Prize for Medical Science (2022).
Reference
| Authors | Atsushi Takahashi-Kanemitsu1,2, Mengxue Lu1, Christopher Takaya Knight1, Takayoshi Yamamoto3,4, Takuo Hayashi5, Yusuke Mii6,7, Takuya Ooki1,8, Ippei Kikuchi1,8, Akira Kikuchi9,10, Nick Barker 11,12,13, Etsuo A. Susaki2, Masanori Taira3,14, and Masanori Hatakeyama1,8,15 |
| Title of original paper | The Helicobacter Pylori CagA Oncoprotein Disrupts Wnt/PCP Signaling and Promotes Hyperproliferation of Pyloric Gland Base Cells |
| Journal | Science Signaling |
| DOI | 10.1126/scisignal.abp9020 |
| Affiliations | 1Department of Microbiology, Graduate School of Medicine, The University of Tokyo, Japan. 2Department of Biochemistry and Systems Biomedicine, Juntendo University Graduate School of Medicine, Japan. 3Department of Biological Sciences, Graduate School of Science, The University of Tokyo, Japan. 4Department of Life Sciences, Graduate School of Arts and Sciences, The University of Tokyo, Japan. 5Department of Human Pathology, Juntendo University Graduate School of Medicine, Japan. 6National Institute for Basic Biology and Exploratory Research Center on Life and Living Systems (ExCELLS), National Institutes of Natural Sciences, Japan. 7Japan Science and Technology Agency, PRESTO, Japan. 8Laboratory of Microbial Carcinogenesis, Institute of Microbial Chemistry, Microbial Chemistry Research Foundation, Japan. 9Department of Molecular Biology and Biochemistry, Graduate School of Medicine, Osaka University, Japan. 10Center for Infectious Disease Education and Research (CiDER), Osaka University, Japan. 11Institute of Molecular and Cell Biology (IMCB), Agency for Science, Technology and Research (A*STAR), Singapore. 12Department of Physiology, Yong Loo Lin School of Medicine, National University of Singapore, Singapore. 13Division of Epithelial Stem Cell Biology, Cancer Research Institute, Kanazawa University, Japan. 14Department of Biological Sciences, Faculty of Science and Engineering, Chuo University, Japan. 15Research Center of Microbial Carcinogenesis, Institute for Genetic Medicine, Hokkaido University, Japan. |