Topics
>RESEARCH
Antibodies Targeting Immunoglobulin E Cε2 Region as Potential Rapid Anti-Allergy Therapy
Researchers identify novel Fab antibody fragments that remove mast cell-bound IgE, enabling faster treatment of severe allergic diseases
Several severe allergic diseases are driven by immunoglobulin E (IgE) bound to immune cells, such as mast cells. Current therapies have delayed effect in these diseases as they can only eliminate free IgE in the serum. Researchers have now identified antibody fragments that target Cε2 domain of IgE, capable of destabilizing its complex with the receptor FcεRI and removing it from mast cells. The antibody fragments offer a potential rapid-acting therapy for IgE-driven allergic diseases.
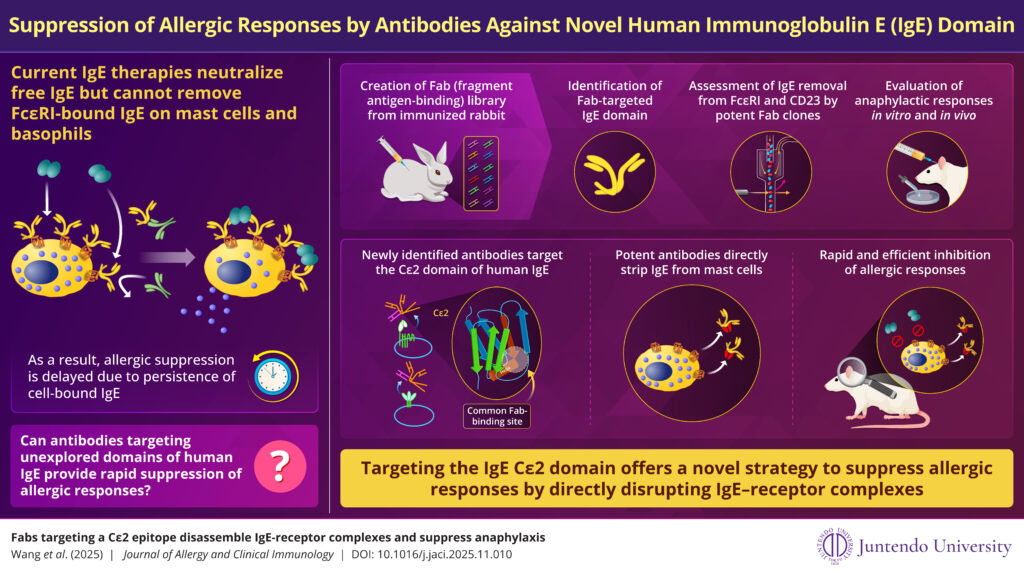
Image title: Antibody Fragments Targeting Human Cε2 Domain Strips IgE from Mast Cells, Rapidly Suppressing Allergic Reactions
Image caption: Researchers identify antibody fragments capable of removing immunoglobulin E bound to mast cells, revealing a potentially fast-acting therapy for severe allergic diseases
Image credit: Tomoaki Ando of Juntendo University
License type: Original content
Usage restrictions: You are free to share and adapt the Infographic material but attribution is required, with a link to the news source.
Allergic diseases represent a major global health burden, placing significant strain on healthcare systems worldwide. Severe conditions such as anaphylaxis, asthma, food allergy, and allergic rhinitis are driven by immunoglobulin E (IgE), an antibody that binds to immune cells including mast cells and basophils. When IgE remains attached to these cells, it sustains exaggerated immune responses to allergens. Although current anti-allergy therapies can neutralize free IgE in the bloodstream, they cannot efficiently remove IgE already bound to immune cells, delaying the onset of clinical benefit.
IgE contains a unique immunoglobulin-like domain, Cε2, which stabilizes its interaction with the high-affinity receptor FcεRI that anchors IgE to the surface of mast cells and basophils. In a recent study published in The Journal of Allergy and Clinical Immunology on December 11, 2025, researchers led by Associate Professor Tomoaki Ando and Professor Jiro Kitaura at Juntendo University Graduate School of Medicine, in collaboration with Toshiaki Maruyama of Abwiz Bio Inc., identified Fab (fragment antigen-binding) antibody fragments that target the IgE Cε2 domain and disrupt the IgE–FcεRI complex, effectively stripping IgE from mast cells.
“Previous studies in mice suggested that targeting the Cε2 domain could suppress IgE-dependent mast cell activation, yet whether this strategy could be applied to human IgE remained unclear due to substantial species differences between human and murine IgE. This gap in knowledge prompted us to ask whether the human IgE Cε2 domain could be exploited as a novel therapeutic target to destabilize IgE–receptor interactions,” explains Dr. Ando.
To systematically investigate antibodies against the human IgE Cε2 domain, the researchers generated Fab antibody libraries from immunized rabbits and screened them for activity against pre-formed IgE–FcεRI complexes. This approach revealed marked differences among Fab clones in their ability to destabilize IgE–receptor interactions. The most potent Fab fragments efficiently removed IgE already bound to mast cell surface receptors.
The team further evaluated whether these Fab antibody fragments could suppress IgE-driven allergic reactions in FcεRI-humanized mouse models. Their experiments showed that the most effective Fab clones significantly reduced allergic responses and inflammation in vivo, demonstrating both cellular and physiological efficacy.
“The study demonstrates, for the first time, that the Cε2 domain of human IgE is a viable therapeutic target, and identifies multiple antibody fragments that show rapid efficacy in cellular assays and in vivo anaphylaxis models,” explains Dr. Ando. “The most immediate real-world application of our findings is the development of a next-generation antibody therapy for allergic diseases that require rapid and reliable symptom control. In addition, this strategy could be applied in clinical settings where temporary but rapid desensitization is needed, such as before allergen exposure during immunotherapy or medical procedures,” he adds.
IgE circulates at low levels in human serum, enabling efficient neutralization of free IgE by existing therapies. However, their inability to remove IgE already bound to mast cells has remained a key limitation in achieving rapid symptom control. Antibody-based strategies targeting the human Cε2 domain may help overcome this challenge by directly destabilizing IgE–receptor complexes, although further studies will be required to confirm their clinical safety and efficacy.
***
Reference
|
Authors |
Hexing Wang1,2, Tomoaki Ando1, Toshiaki Maruyama3, Shigeru CJ Okumura3, Kumi Izawa1, Ayako Kaitani1, Akie Maehara1, Risa Yamamoto1, Paul D Entzminger3, Jonathan K Fleming3, Nobuhiro Nakano1, Keiko Maeda1, Hideo Yagita4, Hideoki Ogawa1, Ko Okumura1, and Jiro Kitaura2 |
|
Title of original paper |
Fabs targeting a Cε2 epitope disassemble IgE-receptor complexes and suppress anaphylaxis |
|
Journal |
The Journal of Allergy and Clinical Immunology |
|
DOI |
|
|
Affiliations |
1Atopy (Allergy) Research Center, Juntendo University Graduate School of Medicine, Japan 2Department of Science of Allergy and Inflammation, Juntendo University Graduate School of Medicine, Japan 3Abwiz Bio, Inc., San Diego, U.S.A. 4Department of Immunology, Juntendo University Graduate School of Medicine, Japan |
***
About Associate Professor Tomoaki Ando
Dr. Tomoaki Ando is an Associate Professor at the Atopy (Allergy) Research Center, Juntendo University Graduate School of Medicine in Tokyo, Japan. He holds both an MD and a PhD from the University of Tokyo. His research spans pediatrics, allergology, and immunology, focusing on the cellular and molecular mechanisms underlying allergic disease. Dr. Ando studies mast cell biology, IgE-mediated allergic responses, allergen cross-reactivity, and inflammatory pathways, aiming to translate fundamental immunological insights into innovative therapeutic strategies for allergic and inflammatory disorders. His work bridges basic science with relevant translational applications.