Topics
>RESEARCH
A Genetic Key to Understanding Mitochondrial DNA Depletion Syndrome
Researchers identify a novel genetic cause of a rare mitochondrial disorder, revealing mechanisms for improved diagnosis and treatment
Mitochondrial diseases, often stemming from complex genetic factors, impact the mitochondria’s energy production. Japanese researchers have now linked MICOS10 gene variants to mitochondrial diseases for the first time. This discovery shows that defects in the MICOS complex disrupt mitochondrial structure and function, offering new insights into the genetic basis of these disorders. Their findings underscore the potential for improved genetic diagnostics and therapeutic approaches targeting mitochondrial function in affected patients.
Role of MICOS10 Variants in Hepatocerebral Mitochondrial DNA Depletion Syndrome
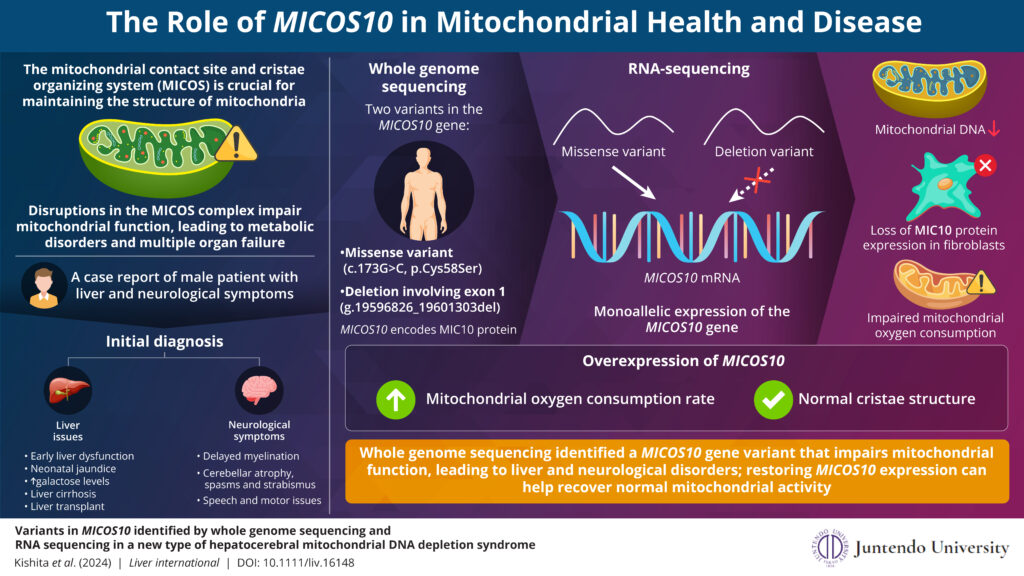
Image caption: Identifying MICOS10 variants enhances our understanding of mitochondrial function and genetic diagnosis in rare diseases
Image credit: Yasushi Okazaki from Juntendo University, Japan
License type: Original content
Usage restrictions: Cannot be reused without permission.
Mitochondrial DNA depletion syndrome (MTDPS) is a rare genetic disorder characterized by a marked decrease in mitochondrial DNA (mtDNA). This condition can cause symptoms including muscle weakness, fatigue, and neurological issues, particularly affecting the liver and brain in cases of hepatocerebral MTDPS. Mitochondrial diseases, which represent some of the most common types of metabolic disorders, can result in the failure of multiple organ systems. Currently, over 400 genes linked to these diseases have been identified. Notably, many of these genes are associated with the mitochondrial contact site and cristae organizing system (MICOS) complex, highlighting the complexity of the genetic factors at play.
In a notable study published in Liver International on November 07, 2024, researchers led by Professor Yasushi Okazaki from the Division of Diagnostics and Therapeutics of Intractable Diseases at Juntendo University, Japan, made important advancements in understanding MTDPS. The research team, including Dr. Kei Murayama, Dr. Yoshihito Kishita, and Dr. Ayumu Sugiura, utilized a combination of whole genome sequencing and RNA sequencing techniques to identify a specific variant in the MICOS10 gene in a patient with the disease. “This is the first report of MICOS10 variants in hepatocerebral MTDPS. Understanding how defects in this complex affect cristae formation and mitochondrial function could provide new insights into the molecular pathogenesis underlying this disease,” explains Prof. Okazaki.
The study focused on a young patient who presented with severe liver dysfunction, including cirrhosis and developmental delays. Despite undergoing a liver transplant, the patient continued to experience neurological symptoms. Laboratory tests revealed defects in the mitochondrial respiratory chain, alongside a significant reduction in mtDNA levels. When the mtDNA in the liver tissue removed during the transplant was quantified, it was found to be only 23.7% of the normal level, leading to a definitive diagnosis of MTDPS.
Further whole genome sequencing found two variants in the MICOS10 gene that likely explained the patient’s symptoms: one was a single nucleotide missense mutation, and the other was a deletion in large exon 1. Although both copies of the gene were present, only the copy with the missense mutation was active, as the exon 1 deletion prevented expression of the other copy. This limited expression from only one deleterious variant of MICOS10 likely disrupted mitochondrial function, contributing to the patient’s condition.
Functional studies conducted in patient-derived fibroblast cells showed that restoring MICOS10 expression improved mitochondrial respiration, evidenced by increased oxygen consumption, and rescued abnormalities of the cristae structures in patient’s fibroblasts. This research confirmed the crucial role of MICOS10 in maintaining mitochondrial structure and function.
The findings from this study represent a major step forward in the understanding of mitochondrial diseases. By illuminating the role of MICOS10, the research opens up potential new avenues for genetic testing and therapeutic development. Prof. Okazaki emphasized that “Clarifying genetic variants that were previously undetectable could greatly improve the efficiency of diagnosis for patients with mitochondrial disorders.”
These groundbreaking insights could unlock targeted treatments, offering hope for restoring mitochondrial function in affected patients. By deepening our understanding of MTDPS and highlighting the power of advanced genetic diagnostics, this research paves the way for a brighter future, bringing renewed optimism to those grappling with these complex disorders.
***
Reference
|
Authors |
Yoshihito Kishita1,2, Ayumu Sugiura2, Nanako Omichi1, Masaru Shimura3, Yukiko Yatsuka2, Kohta Nakamura2, Toju Tanaka4, Mitsuru Kubota5, Kei Murayama2,3, Akira Ohtake6,7, and Yasushi Okazaki2,8. |
|
Title of original paper |
Variants in MICOS10 identified by whole genome sequencing and RNA sequencing in a new type of hepatocerebral mitochondrial DNA depletion syndrome |
|
Journal |
Liver International |
|
DOI |
|
|
Affiliations |
1 Department of Life Science, Faculty of Science and Engineering, Kindai University, Osaka, Japan 2 Diagnostics and Therapeutic of Intractable Diseases, Intractable Disease Research Center, Graduate School of Medicine, Juntendo University, Tokyo, Japan 3 Department of Metabolism, Chiba Children’s Hospital, Chiba, Japan 4 Department of Pediatrics, National Hospital Organization Hokkaido Medical Center, Sapporo, Japan 5 Department of General Pediatrics and Interdisciplinary Medicine, National Center for Child Health and Development, Tokyo, Japan 6 Department of Pediatrics and Clinical Genomics, Saitama Medical University, Moroyama, Saitama, Japan 7 Center for Intractable Diseases, Saitama Medical University Hospital, Moroyama, Saitama, Japan 8 Laboratory for Comprehensive Genomic Analysis, RIKEN Center for Integrative Medical Sciences, Kanagawa, Japan |
***
About Professor Yasushi Okazaki
Professor Yasushi Okazaki is a distinguished expert in genomic medicine, focusing on diagnostics and therapies for intractable diseases. Since 2017, he has served as Professor and Director at Juntendo University Graduate School of Medicine and the Intractable Disease Research Center. With over 30 years of experience, Prof. Okazaki has authored more than 212 original articles, advancing the fields of functional genomics, systems medicine, and comprehensive genome analysis. His notable achievements include pioneering work in translational research and genomic exploration, making him a key contributor to the scientific understanding and treatment of complex diseases.