Realizing genome-based personalized medicine
Bringing about a paradigm shift in psychiatry
―― What are some advantages of having your lab at Juntendo University?
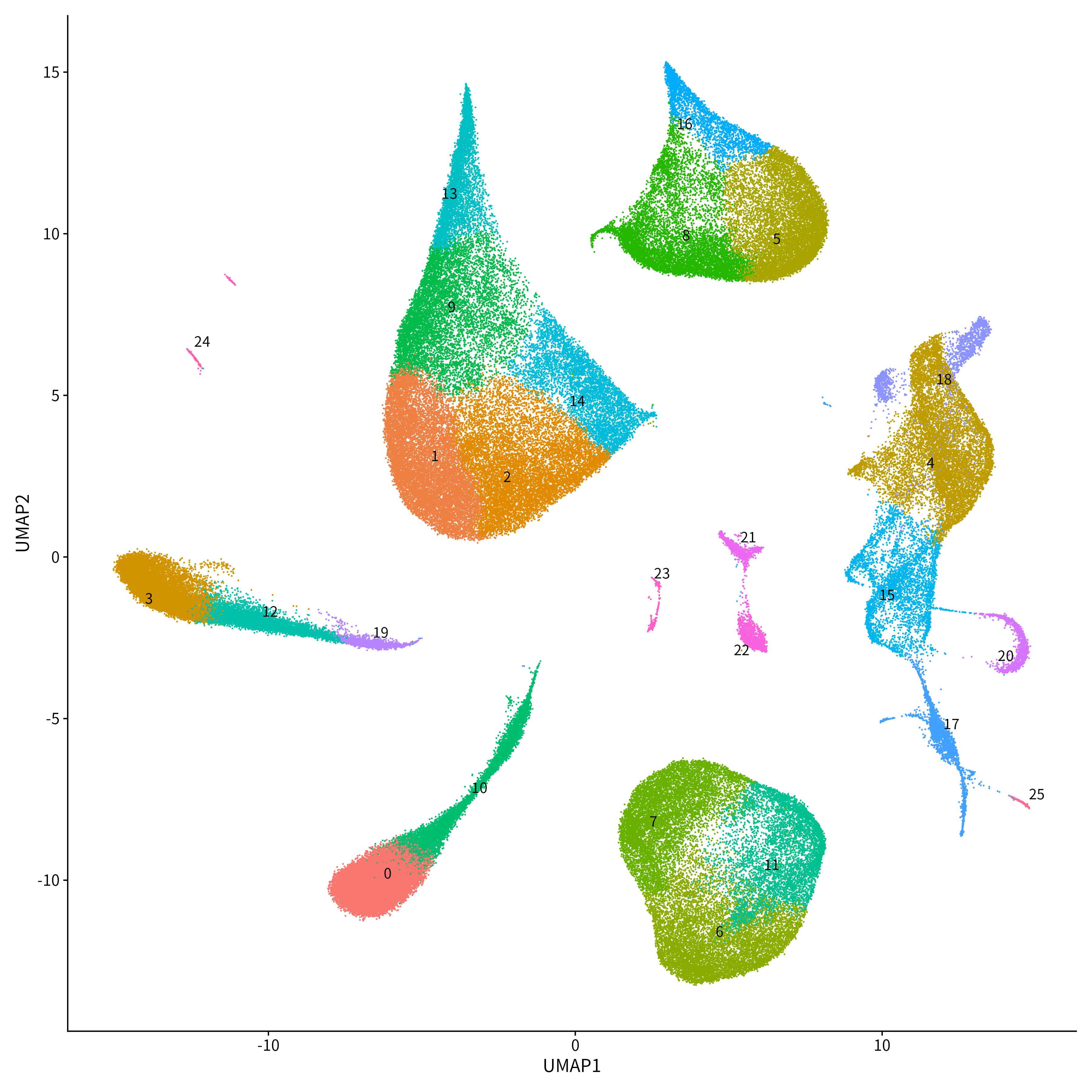
Nishioka: A big advantage is the ability to verify the results of genome analysis using data from many patients, which was not possible at RIKEN.
Kubota-Sakashita: We are verifying the effectiveness of the screened compounds using iPS cells derived from patients with mitochondrial DNA mutations. At Juntendo University, we have the advantage of being able to obtain patient samples, and are also receiving technical training in iPS cell production through the cooperation of the Center for Genomics and Regenerative Medicine, which will support the success of our efforts. Although we are still in the early stages, I believe that cultivating miniature organs, known as organoids, will allow us to make even more significant discoveries.
――As a laboratory, what new goals are you working toward?
Kato: Our primary goal is to definitively establish the paraventricular thalamic nucleus as the cause of bipolar disorder and to develop a method for detecting mitochondrial dysfunction in this region within the next five years. We are also pursuing research into drug discovery, to create personalized treatment tailored to each patient’s condition. While molecular-targeted drug therapies based on genomic diagnosis are commonly used in cancer treatment, psychiatry lags behind by 10 or 20 years. We will continue our work with Dr. Nishioka and Dr. Kubota-Sakashita, to take the first step toward advancing psychiatry in a similar direction.
――That would bring about a major change in psychiatry, wouldn’t it?
Kato: Currently diagnosis in psychiatry primarily involves consultations based on conversation, with diagnoses made according to symptom classification, followed by medication prescriptions. This approach treats mental illnesses as disorders of the mind. However, as our understanding deepens and we recognize that mental illnesses are brain diseases, I believe that, in the future, mental illnesses will also be reclassified based on their pathology at the cellular and molecular levels, informed by genome analysis. While it may take some time to reach that point, I believe a significant paradigm shift in psychiatry is inevitable.