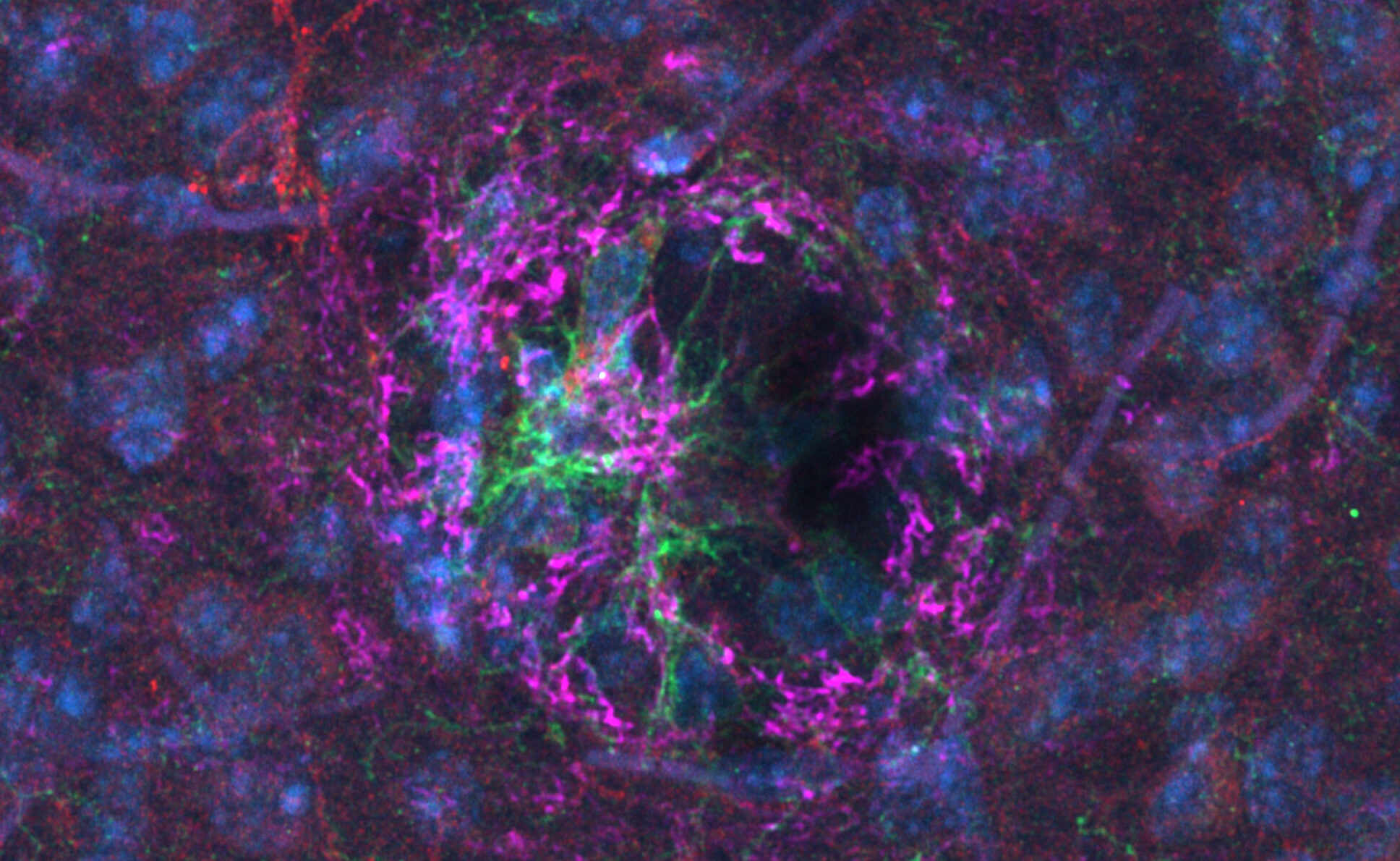
Leading global research on senescent cells linked to aging-related diseases
Juntendo University Graduate School of Medicine, Department of Cardiovascular MedicineDr. Tohru Minamino

Leading global research on senescent cells linked to aging-related diseases
As contemporary society continues to age, there is a growing global interest in developing innovative treatments for age-related conditions such as diabetes and Alzheimer’s disease. A research team led by Professor Tohru Minamino from the Department of Cardiovascular Medicine at Juntendo University Graduate School of Medicine has focused on “senescent cells” as a key contributor to the onset of these diseases. In 2021, the group successfully developed a senolytic vaccine—which achieves the removal of senescent cells—with their findings published in the online edition of Nature Aging. This breakthrough has drawn international attention, driving research and development efforts for senescent cell-targeting therapies worldwide. We had the privilege of speaking with Professor Minamino, a leading expert in this field.

Metabolic stress induced by aging and obesity is a well-known contributor to the onset and progression of age-related diseases, including lifestyle-related conditions and Alzheimer’s disease. However, the underlying mechanisms linking these factors remain largely unclear. For over three decades, our research group has focused on “senescent cells” as a central theme in investigating the mechanisms underlying age-related diseases. Our findings have demonstrated that aging and stress lead to the accumulation of senescent cells in tissues, which results in chronic inflammation, contributing significantly to the onset and progression of these diseases.
The paper titled “Development of a senolytic vaccine targeting GPNMB,” published online in Nature Aging in December 2021, has garnered significant interest both in Japan and internationally. Following its release, our research has attracted numerous offers of support from overseas venture capital firms. Simultaneously, research on senescent cells has seen a surge in activity, with an increasing number of researchers participating in the development of senolytic vaccines and therapeutic agents, establishing this as a highly competitive research area.
Here, we will discuss current progress in senolytic vaccine development, the parallel progress in senolytic drug research, and the establishment of guidelines for cellular senescence studies.


Previous studies have demonstrated that removing accumulated senescent cells can alleviate characteristics of pathological aging associated with age-related diseases. The selective elimination of senescent cells, known as “senolysis,” has led to the development of various senolytic drugs. However, many of these drugs are repurposed anti-cancer agents, raising concerns about their potential side effects. To address this, we aimed to achieve senolysis through a vaccine, which led to the development of the “senolytic vaccine targeting GPNMB.”
GPNMB, or glycoprotein non-metastatic melanoma B, is an aging-associated antigen specifically expressed in senescent cells. An antigen serves as a molecular marker that helps the immune system recognize pathogens or viruses—in this case, senescent cells. A comprehensive analysis of the genetic information of senescent human vascular endothelial cells led to the identification of GPNMB as an aging antigen.
At the core of the senolytic vaccine is a peptide sequence derived from the GPNMB protein. Administration of this peptide stimulates the body to produce antibodies against GPNMB, enabling the immune system to selectively eliminate senescent cells. This is analogous to the mechanism is used in anti-cancer peptide vaccines.
When the vaccine was administered to obese mice, it successfully led to the removal of senescent cells that had accumulated in visceral fat due to obesity. This resulted in a reduction in chronic inflammation within adipose tissue and improved glucose metabolism. Additionally, administering the vaccine to a premature aging model demonstrated an extension of lifespan. Comparative experiments with existing senolytic drugs also showed that the senolytic vaccine caused fewer side effects.
Recently, alongside these advancements, we have been working on the development of RNA vaccines for senolytic applications. Following the global shift towards RNA vaccine technology after the spread of COVID-19, our focus has also transitioned to this approach. Currently, we are conducting experiments using humanized mice containing human senescent cells. These RNA vaccines function similarly to the RNA vaccines used in cancer immunotherapy, leveraging the immune system to target and eliminate senescent cells.

In addition to senolytic vaccines, new research is also being conducted on senolytic drugs. Our research group has demonstrated via mouse experiments that SGLT2 inhibitors (*1), commonly used to treat diabetes, can function as senolytic drugs. As mentioned earlier, many existing senolytic drugs are repurposed anti-cancer medications, raising concerns about potential side effects. However, if SGLT2 inhibitor-based senolytics prove effective, these concerns may be mitigated.
It has long been recognized that calorie restriction can extend human lifespan. Experiments in mice have also confirmed this by showing that calorie restriction suppresses the accumulation of senescent cells associated with aging. Building on this, our research group focused on SGLT2 inhibitors, a class of drugs used to treat diabetes. We hypothesized that by promoting sugar excretion in urine and lowering blood sugar levels, these drugs could mimic the effects of calorie restriction. This, in turn, could prevent the accumulation of senescent cells and promoting their clearance.
To test this hypothesis, we administered SGLT2 inhibitors to mice with high-fat-diet-induced obesity. The treatment successfully induced senolysis, reduced inflammation in visceral fat, and improved glucose metabolism and insulin resistance. To investigate the underlying mechanism, we conducted a comprehensive analysis of metabolites, which revealed that SGLT2 inhibitors increase the levels of a metabolite called AICAR, that activates the phosphorylation enzyme AMPK (*2).
We found that elevated levels of AICAR promote senolytic activity by activating AMPK in senescent cells while suppressing the immune checkpoint molecule PD-L1 (*3). PD-L1 typically inhibits immune cell activity, and its suppression leads to enhanced immune cell activity, further promoting senolysis.
Administration of SGLT2 inhibitors has also been shown to reduce frailty associated with aging and extend the lifespan of mice with progeria. In the future, this promising senolytic drug is expected to be evaluated for its effectiveness in various age-related diseases, including Alzheimer’s disease, and may ultimately be developed for clinical application in humans.


Following the publication of our paper in Nature Aging in December 2021, there has been an increase in new entrants into the field of cellular aging research, resulting in numerous instances of similar validation checks across submitted papers. To address this, our research group, in collaboration with leading experts in the field, including Dr. Marco DeMaria of the European Institute for the Biology of Aging, Dr. Johannes Grillari, and Dr. Mikolaj Ogrodnik of the Ludwig Boltzmann Institute for Traumatology—has developed a set of guidelines to support rigorous and standardized research on cellular aging.
These guidelines establish a standardized set of markers and techniques essential for studying senescent cells in a natural tissue environment. Additionally, they emphasize the importance of expanding research on cellular aging beyond mouse models to include other organisms, integrate omics analysis, and leverage artificial intelligence in these studies.
We hope that, by providing a structured framework for experimental design and evaluation, more researchers will conduct robust and reproducible studies, leading to further advancements in senescent cell research. This work was published in the online edition of Cell on August 8, 2024.

This series of research aims to develop new treatments for age-related diseases—a longstanding medical challenge —by conducting clinical studies focused on the selective removal of senescent cells. Given Japan’s rapidly aging society, this approach has significant public health implications. In experiments on mouse models, senolytic treatment has shown efficacy for addressing diseases such as arteriosclerosis, diabetes, heart failure, and respiratory diseases, as well as for conditions with high “unmet medical needs,” including Alzheimer’s disease, sarcopenia, and frailty.
With respect to senolytic vaccines, we will continue to pursue research on both peptide and RNA vaccines in parallel. Additionally, for new senolytic drugs based on SGLT2 inhibitors, we aim to accelerate their clinical application by leveraging our network with Juntendo University Hospital and other affiliated medical institutions. To further support these efforts, we are in the process of securing external funds from overseas venture capital firms that recognize the societal impact of senolytic therapies. Our ultimate goal is to establish a university-based venture company that can support advancements in these innovative treatments.
*1: SGLT2 inhibitors: SGLT2 is a sodium-glucose cotransporter located in the proximal tubules of the kidney. Inhibiting this transporter helps treat diabetes by allowing glucose to be excreted in the urine.
*2: AMPK: AMP-activated protein kinase, an enzyme that is activated when there is an energy deficiency within the cell. It is activated by AMP and its metabolic analog, AICAR.
*3: PD-L1: Programmed cell death ligand 1, a protein expressed on the surface of cells that binds to PD-1, a receptor on T cells (immune cells), thereby suppressing immune cell activity and acting as a brake to prevent immune cells from attacking the body.


Professor, Juntendo University Graduate School of Medicine, Department of Cardiovascular Medicine
Dr. Tohru Minamino graduated from Chiba University School of Medicine in 1989 and completed his clinical training, following which he earned a doctorate in medicine from the University of Tokyo School of Medicine. After his research fellowship at Harvard Medical School, in 2012 he was appointed Professor at the Department of Cardiovascular Biology and Medicine, Niigata University Graduate School of Medical and Dental Sciences. In 2020, he joined Juntendo University Graduate School of Medicine as a Professor in the Department of Cardiovascular Biology and Medicine.
Professor Minamino is an expert in the field of cardiovascular medicine, with a special focus on arteriosclerosis and heart failure. His research on aging and cardiovascular regeneration has been published in high-impact journals such as Nature, Nature Medicine, Nature Genetics, Nature Cell Biology, Cell, Cell Metabolism, and The Lancet. As a recognition of his contributions to medical research, Professor Minamino has received several prestigious awards, like the Japan Circulation Society Award (Sato Award), the Bälz Award, and the Japan Medical Association Research Encouragement Award.
He leads several national and international academic societies of internal medicine, cardiovascular science, and aging, besides heading multiple research groups with experts in basic and clinical medicine. He actively contributes to Japan’s scientific research ecosystem and serves as an academic investigator for the Ministry of Education, Culture, Sports, Science and Technology. Additionally, he also conducts research at the Japan Society for the Promotion of Science’s Center for Research on Science Systems, and is a field advisor and a program officer for AMED-CREST/PRIME and AMED, respectively.
Researchmap