“Bicarbonate Ion Biology”: Pioneering New Treatments for Cerebral Infarction and Other Conditions
Department of Biochemistry, Graduate School of Medicine, Department of Biochemistry, Juntendo University, Contract Lecturer Dr. Airi Jo-Watanabe

“Bicarbonate Ion Biology”: Pioneering New Treatments for Cerebral Infarction and Other Conditions
The body’s acid-base balance, which regulates pH levels, is crucial for sustaining life. Research has shown that fluctuations in this balance can significantly affect cellular function. Dr. Airi Jo-Watanabe, an associate professor in the Department of Ion Signaling and Response, The Sakaguchi Laboratory, Keio University School of Medicine, and a contract lecturer in the Department of Biochemistry at Juntendo University Graduate School of Medicine, has identified the mechanism by which bicarbonate ions trigger cellular responses. These findings could lead to new treatments for cerebral infarction and other disorders.

Bicarbonate ions (HCO₃⁻) regulate the body’s acid-base balance, ensuring stable pH levels in tissues and organs. These anions result from the ionization of a hydrogen molecule from carbonic acid (H₂CO₃) and contribute to the acid-base balance through the bicarbonate buffer system.
Changes in cell behavior due to acid-base balance have traditionally been explained by shifts in pH, determined by proton (hydrogen ion, H⁺) concentration. pH and ion levels are critical environmental factors that significantly influence cellular function. In the body, pH is maintained at approximately 7.4 through multiple buffer systems, including the bicarbonate buffer system. This regulatory process is known as pH homeostasis.
Earlier studies showed that several G protein-coupled receptors (GPCRs) detect protons and trigger cellular responses when proton concentrations rise (or when pH decreases). More recent research indicates that bicarbonate ions and carbon dioxide can also elicit cellular responses, suggesting receptors that recognize these molecules.
While investigating the mechanisms of acid-base balance, our research group discovered that the GPCR receptor ‘GPR30’ increases intracellular calcium levels in response to bicarbonate ions, independent of pH changes. Although acid-base balance is crucial for sustaining life, its role in cell signaling remains underexplored. Our findings provide new insights into this unexplored aspect of cellular regulation.


These membrane proteins, including GPCRs, are essential for detecting external signals and transmitting them into the cell. Among the 800 human GPCRs identified so far, half of which are olfactory receptors, my focus was on GPR30, a receptor initially studied for its role in estrogen signaling. However, much about its function remained unclear, and I aimed to uncover its true nature.
This research took an unexpected turn due to a chance discovery during an experiment. While testing whether GPR30 could be activated by estrogen or other ligands, we accidentally broke a bottle of buffer and ran out of it. To continue the experiment, we diluted some ligand candidates in a phenol red-free medium. Surprisingly, we found that the cells were activated by the medium itself.
To identify the cause of receptor activation, we systematically added each component of the medium one at a time while monitoring cellular responses. Amino acids, glucose, and vitamins had no effect, but the cells responded to a mixed solution of inorganic salts. By systematically eliminating individual elements, we pinpointed bicarbonate ions as the trigger for GPR30 activation. This discovery revealed a novel signaling mechanism distinct from the conventional activation of GPCRs by protons.

GPCRs were traditionally believed to be activated only by more complicated specific ligands (binding molecules), such as hormones and neurotransmitters. Then, in the early 2000s, researchers wondered whether some GPCRs could sense protons. Our research has now shown that bicarbonate ions, which were previously considered to be mere environmental factors, can also activate GPCRs. This finding introduces a new concept: changes in acid-base balance can drive cellular responses through GPCR activation.
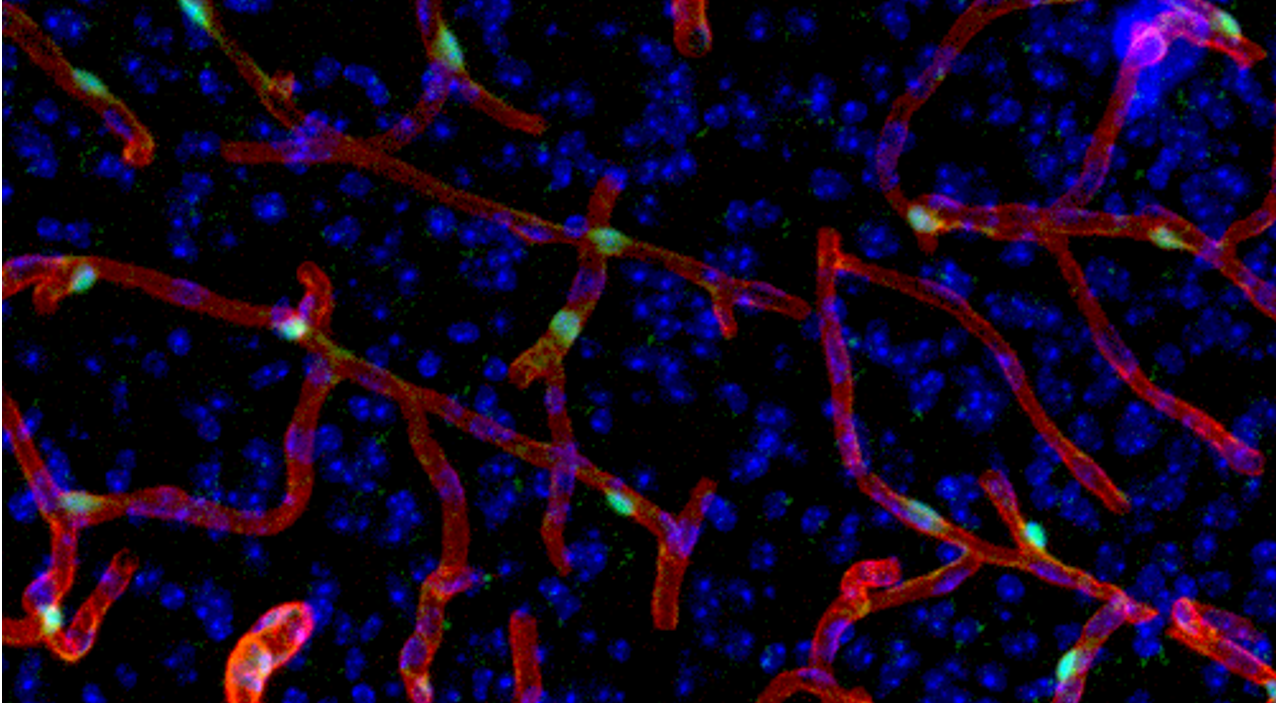
Additionally, we found that GPR30 is expressed in pericytes, the wall cells of brain microvasculature, where it helps regulate blood flow. This suggests that GPR30 may be implicated in ischemia-reperfusion injury.
Restoring blood flow is critical in treating cerebral infarction, but recanalization can further damage neurons weakened by ischemia. Additionally, vascular inflammation and oxidative stress following recanalization can contribute to nerve cell damage. Rapidly restoring blood flow to protect neurons from ischemia is believed to aid recovery. GPR30 could serve as a new therapeutic target for cerebral infarction by regulating this process.
To explore GPR30’s role in the pathology of cerebral infarction, we conducted experiments using a cerebral infarction model with knockout mice lacking GPR30. Mice without GPR30 showed less damage after occlusion and reopening of the left middle cerebral artery compared to normal mice, suggesting GPR30 contributes to the exacerbation of ischemia-reperfusion injury.
Reducing acute ischemia-reperfusion injury is critical in treating cerebral infarction. Focusing on bicarbonate ions and clarifying the mechanisms underlying cerebral ischemia-reperfusion injury could lead to groundbreaking treatments.


We are currently developing drugs that modulate GPR30 activity. In our laboratory, we have screened compounds in cultured cells to identify those that regulate GPR30 activity. Moving forward, we plan to verify the effects in mouse models and develop them into therapeutic drugs for cerebral infarction and other diseases.
Our research could contribute to the treatment of other diseases beyond cerebral infarction. The concentration of bicarbonate ions is a key factor in pathophysiological conditions such as renal and respiratory failure. The kidneys regulate bicarbonate ion excretion, while the lungs maintain acid-base balance by expelling CO2. Investigating the role of GPR30 in these organs could lead to new treatments for these conditions.
This research began with a serendipitous discovery during an experiment, which led to the development of a new concept: “regulation of intracellular signaling by acid-base balance.” My goal is to establish a new research field, “bicarbonate ion biology,” and deepen our understanding of the relationship between acid-base balance and GPCRs. I hope to explore new possibilities in medicine and biology through this work.

Department of Ion Signaling and Response, The Sakaguchi Laboratory, Keio University School of Medicine, Associate Professor
Department of Biochemistry, Graduate School of Medicine, Department of Biochemistry, Juntendo University, Contract Lecturer
Researchmap