Topics
>RESEARCH
From Symptoms to Biology: Neurodegeneration in Paraventricular Thalamus in Bipolar Disorder
Accumulation of neurodegenerative disease-associated proteins plays a key role in the symptoms of bipolar disorder
Mitochondrial dysfunction has been implicated in bipolar disorder (BD). However, it remains unclear which brain region is affected. Now, researchers from Japan have investigated key brain regions involved in mood regulation (BD symptoms), such as the paraventricular thalamus and medial temporal regions; they found granulovacuolar degeneration in the paraventricular thalamus and verified the accumulation of tau proteins in medial temporal region. These findings pave the way for the development of new treatment strategies for BD.
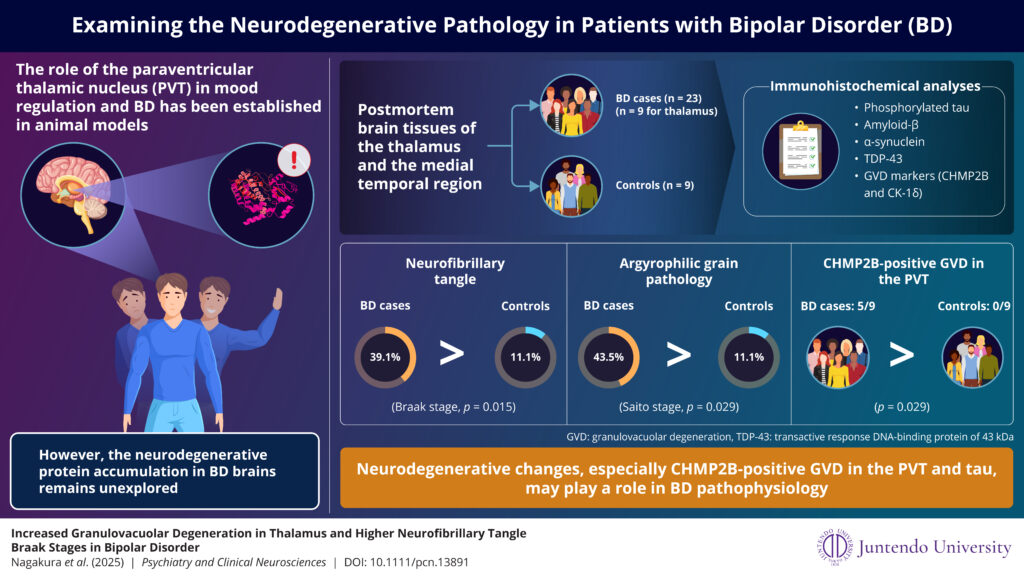
Image title: Neurodegenerative pathology of bipolar disorder
Image caption: Researchers have examined neurodegenerative pathology in the paraventricular thalamus and medial temporal region, including the hippocampus, in patients with bipolar disorder.
Image credit: Prof. Tadafumi Kato from Juntendo University Graduate School of Medicine, Japan
License type: Original content
Usage restrictions: You are free to share and adapt the Infographic material, but attribution is required, with a link to the news source.
Bipolar disorder (BD) is a chronic mental health condition characterized by recurrent episodes of depression and mania. It poses a substantial burden on global health, with an increasing incidence. Despite its prevalence, there exists a significant gap in understanding the underlying neuropathological mechanisms. Although mitochondrial dysfunction has been implicated in BD, the specific brain region damaged is not yet fully understood. A deeper understanding is essential for advancing research efforts and developing better therapeutic strategies.
In an effort to bridge the knowledge gap, a team of researchers in Japan investigated the thalamus and medial temporal regions of the brain—areas involved in mood regulation and cognitive function. The research team, led by Professor Tadafumi Kato from the Department of Psychiatry and Behavioral Science, Juntendo University Graduate School of Medicine, Japan, and Dr. Akito Nagakura from Tokyo Metropolitan Matsuzawa Hospital, Japan, set out with a vision to change the trajectory of BD research. Their findings were published in Psychiatry and Clinical Neurosciences on September 2, 2025.
“Although animal models have pointed to the involvement of the paraventricular thalamic nucleus in the pathophysiology of BD, neuropathological knowledge is limited. In particular, we aimed to investigate whether the accumulation of certain neurodegenerative proteins, previously associated with neurological diseases, might play a role in the pathology of BD,” says Prof. Kato.
The research involved an analysis of postmortem brain tissue, specifically the paraventricular thalamus and medial temporal regions, which were studied in detail. The postmortem tissues were subjected to immunohistochemical analyses using antibodies targeting key neurodegenerative markers, including phosphorylated tau, amyloid β, α-synuclein, and TDP-43. Additionally, markers of granulovacuolar degeneration (GVD), such as CHMP2B and CK-1δ, were investigated. This comprehensive approach enabled an efficient assessment of the expression and distribution of multiple proteins that are commonly associated with neurodegenerative diseases.
The findings revealed significantly higher neurofibrillary tangle (NFT) stages in patients with BD, along with argyrophilic grain pathology. Both are related to tau proteins that accumulate in brain cells and are commonly associated with aging. These findings replicated a greater burden of tau-related pathology in BD, as suggested by previous postmortem brain and neuroimaging studies, possibly linked to the age of onset.
More importantly, the presence of CHMP2B-positive GVD in the paraventricular thalamus in about half of the BD cases was an observation not previously reported.
Together, these insights highlight the potential role of neurodegenerative protein accumulation and dysfunctional paraventricular thalamus in the underlying biology of BD.
Overall, the findings add to a growing body of evidence that suggests that BD is a brain-based disease. By uncovering specific protein pathologies, such as CHMP2B-positive GVD, and tau accumulation in key brain regions, the research strengthens the biological understanding of BD beyond simply clinical symptoms.
As the understanding of underlying brain changes improves, there is a growing need for early detection, personalized treatment strategies, and therapies that address the root mechanisms.
“Our study, by demonstrating the presence of CHMP2B-positive GVD in the paraventricular thalamus and increased NFT stages in patients with BD, may pave the way for the development of new diagnostic tools and targeted therapies,” concludes Prof. Kato.
***
Reference
|
Authors |
Akito Nagakura1,2, Ito Kawakami1,2,3, Araki Kimura1,2,4, Kenji Ikeda1,4, Kenichi Oshima1, Mie Kubota-Sakashita2, and Tadafumi Kato2 |
|
Title of original paper |
Increased Granulovacuolar Degeneration in Thalamus and Higher Neurofibrillary Tangle Braak Stages in Bipolar Disorder |
|
Journal |
Psychiatry and Clinical Neurosciences |
|
DOI |
|
|
Affiliations |
1Tokyo Metropolitan Matsuzawa Hospital, Japan 2Department of Psychiatry and Behavioral Science, Juntendo University Graduate School of Medicine, Japan 3Molecular Pathology and Histology, Tokyo Metropolitan Institute of Medical Sciences, Japan 4Dementia Research Project, Tokyo Metropolitan Institute of Medical Science, Japan |
***
About Professor Tadafumi Kato
Dr. Tadafumi Kato is a Professor at the Department of Psychiatry and Behavioral Science at Juntendo University Graduate School of Medicine, Japan. He graduated from the University of Tokyo in 1988. With over 400 peer-reviewed publications, he proposed the mitochondrial dysfunction hypothesis of bipolar disorder, and his current research focuses on the neurobiological basis of bipolar disorder, encompassing whole genome sequencing, neuroimaging, and the development of mood stabilizers. He has been honored with multiple awards, including the Brain & Behavior Research Foundation Colvin Prize (2017) and Mogens Schou Award for Research (2019).