Topics
>RESEARCH
Structural Adaptations in Aging Podocytes
Juntendo University researchers find that podocytes in aged rats undergo structural remodeling to maintain renal function
Podocytes are specialized cells in the kidneys that do not renew with age. Researchers from Juntendo University used electron microscopy to show that podocytes in older rats are much larger and have significant structural differences from those in young rats. These findings highlight age-related structural changes and could increase the accuracy of staging the progression of glomerular disorders in kidneys.
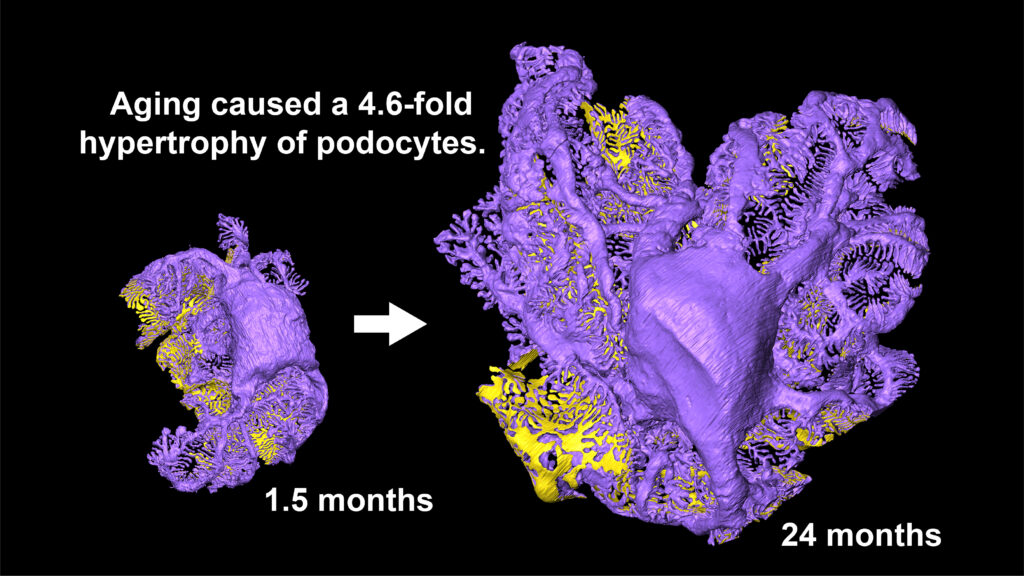
Image title: Whole-cell three-dimensional reconstruction of a podocyte in rats kidney
Image caption: A single podocyte was reconstructed in three dimensions based on serial glomerular section images obtained by array tomography. The three-dimensional reconstruction enabled quantitative volume measurement, revealing that podocytes undergo approximately 4.6-fold hypertrophy with aging.
Image credit: Takashi Amari from Juntendo University, Japan
License type: Original content
Usage restrictions: Credit must be given to the creator.
The kidneys are vital organs that sustain life by filtering the blood and producing urine. This filtration process takes place in specialized structures called glomeruli, where podocytes play a crucial role by forming the filtration barrier on the glomerular surface. Mature podocytes cannot regenerate once lost, which means that the podocytes generated during fetal development must be used throughout life. It is well known that the number of podocytes decreases with age; however, lost podocytes are not replaced by newly generated cells, and continued podocyte depletion ultimately leads to loss of glomerular function. Therefore, the remaining podocytes are thought to adapt in order to preserve glomerular function despite a reduction in cell number; however, how podocytes adapt to this loss has long remained unclear. In this study, the research team employed array tomography (AT), a technique that enables whole-cell observation of podocytes with their complex three-dimensional architecture, to elucidate age-related structural changes in podocytes in rats. Their findings were made available online on December 17, 2025, in the Journal of the American Society of Nephrology.
AT is an advanced imaging technique in which thousands of serial tissue sections are imaged using scanning electron microscopy, enabling high-resolution, three-dimensional observation of relatively large tissue volumes. The research team optimized the standard AT workflow to enable analysis of entire glomeruli, thereby establishing a technique that allows visualization of the complete structure of any individual podocyte within a glomerulus.
In this study, whole-cell three-dimensional reconstructions of podocytes were generated from rats at young (1.5 months of age), adult (6 months of age), and aged (24 months of age) stages. The reconstructed podocytes could be examined on a PC from any desired orientation, and quantitative evaluations, including measurements of cell volume, could be performed.
In the present study, eight distinct types of structural changes associated with podocyte aging were identified. It is known that podocytes are gradually lost with age, resulting in a reduction in cell number; however, this study further demonstrated that portions of podocytes undergo fragmentation and are subsequently lost.
As podocytes are lost, podocyte density on the glomerular surface decreases, while the volume of remaining aged podocytes increases markedly. The volume of aged podocytes was found to be approximately 4.6-fold greater, indicating compensatory hypertrophy in response to podocyte loss. In addition, areas lost through fragmentation were repaired by coverage from surrounding podocytes, during which atypical self-cellular junctions were frequently formed. These autocellular junctions are entirely absent in normal glomeruli and are considered to represent structural “footprints” of injury repair in aging glomeruli.
Furthermore, although aging cells generally exhibit a decline in intracellular degradation capacity for unnecessary cellular components, podocytes were found to compensate for this functional decline by exporting such materials into the extracellular space rather than degrading them intracellularly.
The research team is currently analyzing human podocytes, and preliminary findings indicate that aging-related structural changes in human podocytes are more diverse than those observed in rats. The longer lifespan of humans compared with that of rats is expected to be a key factor in elucidating these structural changes. To characterize the aging process of human podocytes, analyses across a wide age range are required, and additional factors such as sex and the presence of underlying diseases must also be taken into consideration.
The research team has already begun comprehensive analyses of human podocyte aging by utilizing kidney biopsy specimens obtained at the affiliated university hospital. Furthermore, because AT enables observation of entire glomeruli, the team plans to apply this technique in the future to glomerular pathology specimens to analyze low-density or focal lesions that are often overlooked by conventional pathological methods.
***
Reference
|
Authors |
Takashi Amari1*, Takayuki Miyaki1*, Mami Kishi1, Jingyuan Xu1, Makoto Sugiura1, Hisako Kaneda1, Yuta Sakai1, Rhianna Imura1,2, Yuri Takeuchi1,2, Juan Alejandro Oliva Trejo1, Yuto Kawasaki1, Takuya Omotehara1, Takako Negishi-Koga3,4, Muneaki Ishijima3,4, Junji Yamaguchi5, Soichiro Kakuta5, Koichiro Ichimura1,5 *: These authors contributed equally to this work. |
|
Title of original paper |
Structural Plasticity of Aged Podocytes Revealed by Volume Electron Microscopy |
|
Journal |
Journal of the American Society of Nephrology |
|
DOI |
|
|
Affiliations |
1. Department of Anatomy and Life Structure, Juntendo University Graduate School of Medicine, Tokyo, Japan; 2. Medical and Science Course, Hiroo Gakuen Senior High School, Tokyo, Japan; 3. Department of Medicine for Orthopedics and Motor Organ, Juntendo University Graduate School of Medicine, Tokyo, Japan; 4. Department of Community Medicine and Research for Bone and Joint Diseases, Juntendo University Graduate School of Medicine, Tokyo, Japan; 5. Laboratory of Morphology and Image Analysis, Research Support Center, Juntendo University Graduate School of Medicine, Tokyo, Japan. |
***
About Dr. Takashi Amari
Takashi Amari is a Research Fellow at the Department of Anatomy and Life Structure, Juntendo University Graduate School of Medicine, Japan. Dr. Amari received his doctorate in 2024. His work focuses on physical therapy, rehabilitation science, and renal function.
About Dr. Takayuki Miyaki
Takayuki Miyaki is an Assistant Professor at the Department of Anatomy and Life Structure, Juntendo University Graduate School of Medicine, Japan. Dr. Miyaki’s research focuses on cell biology and anatomy, with an emphasis on studying renal structure through electron microscopy.