Topics
>RESEARCH
New Study Uncovers How Altered Gene Expression Can Induce Autism
New Study Uncovers How Altered Gene Expression Can Induce Autism
Researchers show how mutations of gene transcription and chromatin regulation-related genes cause autism.
The loss-of-function mutation of KMT2C, a gene involved in histone modification, leads to the development of autism and other neurodevelopmental deficits. However, the precise mechanism of the disease progression is still unknown. Now, researchers from Japan have developed an animal model and elucidated the mechanism by which mutation in genes involved in chromatin modification causes autism. They have also discovered a drug that can be used in the treatment of autism.
Link between transcriptomic dysregulation and autism spectrum disorder.
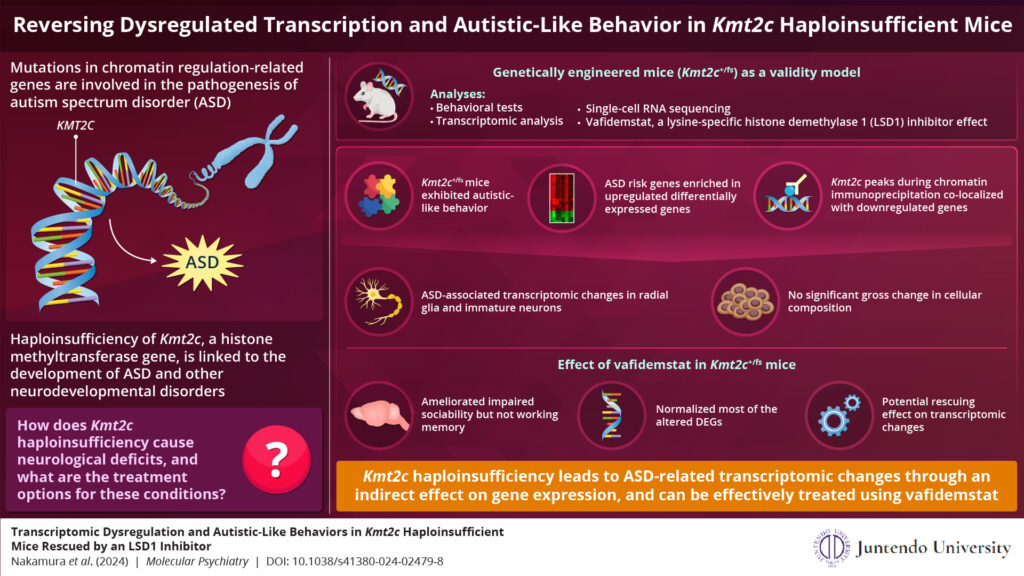
Image caption: Genes involved in chromatin modification and gene transcription are associated with the progression of neurodevelopmental disorders. Researchers from Japan have developed a new mouse model to study the molecular mechanism behind the ability of KMT2C to cause autism spectrum disorder. They also showed that vafidemstat has a rescuing effect by normalizing disrupted gene expression.
Image credit: Tadafumi Kato from Juntendo University, Japan
License type: Original content
Usage restrictions: You are free to share and adapt the Infographic material but attribution is required, with a link to the news source.
Autism spectrum disorder (ASD) encompasses neurodevelopmental conditions where patients display repetitive behavior and impaired sociality. Genetic factors have been shown to influence the development of ASD. Additionally, recent studies have shown that the genes involved in chromatin modification and gene transcription are involved in the pathogenesis of ASD. Among the many genes implicated in this process, the gene KMT2C (lysine methyltransferase 2c), which codes for a catalytic unit of H3K4 (histone H3 lysine 4) methyltransferase complex, has been identified to be associated with the development of autism and other neurodevelopmental disorders. Previous studies have shown that haploinsufficiency (a condition where, of the two copies of the gene, only one remains functional) of KMT2C is a risk factor for ASD and other neurodevelopmental disorders. However, the molecular mechanism through which the loss-of-function mutation in KMT2C leads to these conditions remains unclear.
To address this knowledge gap, researchers from Juntendo University, RIKEN, and the University of Tokyo in Japan aimed to provide answers to these questions in a benchmark study published in the journal Molecular Psychiatry on 26 March 2024. The research team included Professor Tadafumi Kato from the Department of Psychiatry and Behavioral Science at Juntendo University Graduate School of Medicine, Dr. Takumi Nakamura and Dr. Atsushi Takata from the RIKEN Center for Brain Science, and Professor Takashi Tsuboi from Graduate School of Arts and Sciences, The University of Tokyo.
To get to the bottom of KMT2C’s role in ASD pathogenesis, the team developed and analyzed genetically engineered strain mice (Kmt2c+/fs) having a frameshift mutation that models the KMT2C haploinsufficiency. They then performed various behavioral analyses, in which they observed that the mutant mice exhibited lower sociality, inflexibility, auditory hypersensitivity, and cognitive impairments, which are all ASD-related symptoms.
Next, they performed transcriptomic and epigenetic profiling to understand the basis of the molecular changes observed in the mutant mice. What they discovered was remarkable: the genes associated with increased ASD risk showed higher expression in these mutant mice. Dr. Takata exclaims, “This was somewhat unexpected. KMT2C mediates H3K4 methylation, which is thought to activate gene expression, and thereby KMT2C haploinsufficiency was expected to cause reduced expression of target genes.”
To gain mechanistic insights into their finding, the researchers carried out chromatin immunoprecipitation, a technique to determine the location on the DNA where the protein interacts with it. They found an overlap between KMT2C and the differentially expressed genes exhibiting reduced expression, suggesting that KMT2C haploinsufficiency leads to ASD-related transcriptomic changes through an indirect effect on gene expression.
Further, to identify the cell types that contribute more to the pathological changes seen in the mutant mice, the researchers performed single-cell RNA sequencing of newborn mice brains. They observed that the altered genes associated with ASD risk were predominant in undifferentiated radial glial cells. However, a gross change in the cell composition was not observed, implying that the transcriptomic dysregulation does not severely impact cell fate.
Finally, the researchers tested the effects of vafidemstat, a brain penetrant inhibitor of LSD1 (lysine-specific histone demethylase 1A), that could ameliorate histone methylation abnormalities. They found that vafidemstat improved the social deficits in the mutant mice and had an exceptional rescuing effect by changing the expression levels of the differentially expressed genes to their normal expression level. This finding showed that vafidemstat is a valid drug for mutant mice and can potentially help restore the normal transcriptomic state.
What sets this discovery apart is that it challenges the commonly held belief that ASD disability may not be cured and demonstrates the efficacy of vafidemstat in improving ASD-like phenotypes. The results open doors to future research to strengthen the foundation for the pharmacologic treatment of ASD and other neurodevelopmental disorders. Prof. Kato concludes, “Our research shows that drugs similar to vafidemstat may be generalizable to multiple categories of psychiatric disorders.”
***
Reference
|
Authors |
Takumi Nakamura1,2,3,4, Toru Yoshihara5, Chiharu Tanegashima6, Mitsutaka Kadota6, Yuki Kobayashi7, Kurara Honda1, Mizuho Ishiwata2,3, Junko Ueda1,3, Tomonori Hara1,8, Moe Nakanishi9,10, Toru Takumi9,11, Shigeyoshi Itohara7, Shigehiro Kuraku6,12,13, Masahide Asano5, Takaoki Kasahara3,14, Kazuo Nakajima2,3,15, Takashi Tsuboi4, Atsushi Takata1,16, and Tadafumi Kato2,3 |
|
Title of original paper |
Transcriptomic dysregulation and autistic-like behaviors in Kmt2c haploinsufficient mice rescued by an LSD1 inhibitor |
|
Journal |
Molecular Psychiatry |
|
DOI |
|
|
Affiliations |
1Laboratory for Molecular Pathology of Psychiatric Disorders, RIKEN Center for Brain Science, Saitama, Japan. 2Department of Psychiatry and Behavioral Science, Juntendo University Graduate School of Medicine, Tokyo, Japan. 3Laboratory for Molecular Dynamics of Mental Disorders, RIKEN Center for Brain Science, Saitama, Japan. 4Department of Life Sciences, Graduate School of Arts and Sciences, The University of Tokyo, Tokyo, Japan. 5Institute of Laboratory Animals, Kyoto University Graduate School of Medicine, Kyoto, Japan. 6Laboratory for Phyloinformatics, RIKEN Center for Biosystems Dynamics Research, Hyogo, Japan. 7Laboratory for Behavioral Genetics, RIKEN Center for Brain Science, Saitama, Japan. 8Department of Organ Anatomy, Tohoku University Graduate School of Medicine, Miyagi, Japan. 9Laboratory for Mental Biology, RIKEN Center for Brain Science, Saitama, Japan. 10Laboratory for Molecular Mechanism of Brain Development, RIKEN Center for Brain Science, Saitama, Japan. 11Department of Physiology and Cell Biology, Kobe University School of Medicine, Hyogo, Japan. 12Molecular Life History Laboratory, Department of Genomics and Evolutionary Biology, National Institute of Genetics, Shizuoka, Japan. 13Department of Genetics, SOKENDAI (Graduate University for Advanced Studies), Shizuoka, Japan. 14Institute of Biology and Environmental Sciences, Carl von Ossietzky University of Oldenburg, Oldenburg, Germany. 15Department of Physiology, Teikyo University School of Medicine, Tokyo, Japan. 16Research Institute for Diseases of Old Age, Juntendo University Graduate School of Medicine, Tokyo, Japan. |
***
About Takumi Nakamura
Dr. Takumi Nakamura is a Research Scientist in the Laboratory for Molecular Pathology of Psychiatric Disorders at the RIKEN Center for Brain Science, Japan. Dr. Nakamura earned a Ph.D. from The University of Tokyo in 2020, and his research interests include autism spectrum disorder, schizophrenia, and bipolar disorder. He has published 12 peer-reviewed articles since 2016 and has received the 2023 Young Researcher Development Program Encouragement Award from the Japanese Society of Biological Psychiatry.
About Professor Atsushi Takata
Dr. Atsushi Takata is a Principal Investigator and Team Leader of the Laboratory for Molecular Pathology of Psychiatric Disorders at the RIKEN Center for Brain Science, Japan. He earned M.D. and Ph.D. degrees in 2004 and 2011 from Kyushu University. Prof. Takata researches the fundamental biological basis of neuropsychiatric disorders such as schizophrenia, autism spectrum disorders, and mood disorders. He received the Society of Biological Psychiatry Academic Award in 2013 and has published over 70 peer-reviewed articles since 2009.
About Professor Tadafumi Kato
Dr. Tadafumi Kato is a Chief Professor in the Department of Psychiatry and Behavioral Science at Juntendo University Graduate School of Medicine, Japan. He earned an M.D. from the University of Tokyo in 1988 and a Ph.D. from Shiga University of Medical Science in 1995. Prof. Kato researches the neurobiology and genomics of bipolar disorder. He received the 2017 Brain and Behavior Foundation’s Colvin Prize and the 2019 International Society for Bipolar Disorders’ Mogens Schou Award for Research. He has published over 400 peer-reviewed articles since 1990.