Topics
>RESEARCH
A Novel ‘Senolytic’ Strategy for Treating Aging-related Diseases
A Novel ‘Senolytic’ Strategy for Treating Aging-related Diseases
Study by researchers from Japan highlights the senescent cell removal mechanisms of canagliflozin— a sodium glucose co-transporter 2 inhibitor
Aging-related physiological decline and the accumulation of senescent or aging cells in bodily tissues can trigger a number of diseases. The removal of senescent cell, also known as ‘senolysis,’ can therefore, serve as an effective strategy against aging-related diseases. Now, researchers from Japan have uncovered novel molecular mechanisms underlying the senolytic effects of canagliflozin—a sodium glucose co-transporter 2 inhibitor used to control blood glucose levels.
Role of sodium glucose co-transporter 2 inhibitor in preventing age-associated diseases
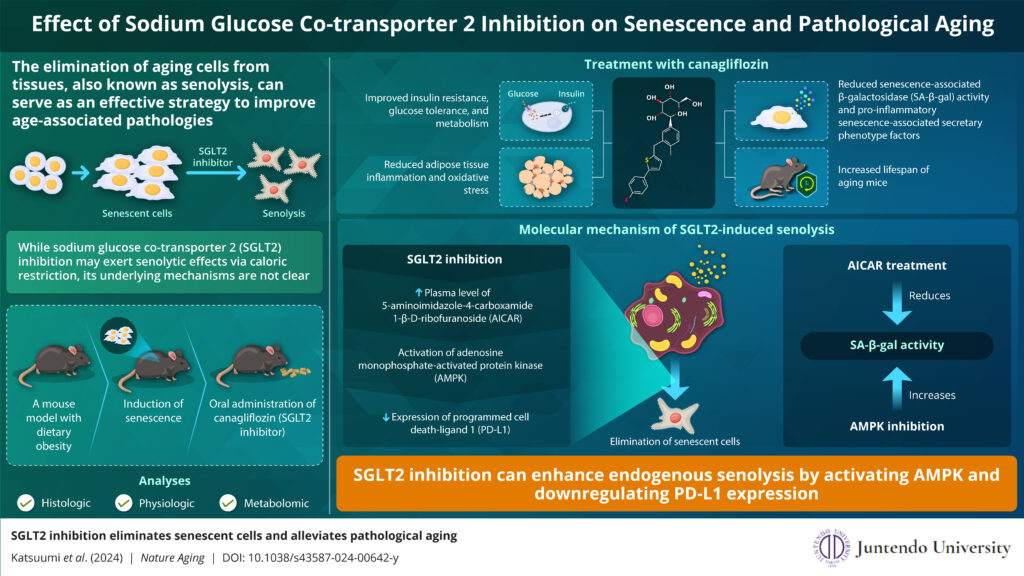
Image caption: Researchers from Juntendo University, Japan examine the molecular mechanisms underlying the senolytic or senescent cell removal effects of the the sodium glucose co-transporter 2 inhibitor and its therapeutic potential in the treatment of aging-related pathologies.
Image credit: Professor Tohru Minamino from Juntendo University, Japan
License type: Original content
Usage restrictions: You are free to share and adapt the Infographic material but attribution is required, with a link to the news source.
The process of aging is accompanied by a decline in physiological functions, which can lead to cardiovascular, neurodegenerative, and metabolic diseases. Aging of cells, also known as ‘cellular senescence’— is a process in which a cell ages and permanently stops dividing but does not die. The accumulation of such ‘senescent’ cells in tissues is then known to contribute to age-associated diseases. Elimination of senescent cells or ‘senolysis’ can, therefore, serve as an effective therapeutic strategy for the improvement of physiological function and prevention of aging-related diseases. However, conventional senolytic agents which directly inhibit cellular aging signals, can have concerning and long-term side effects. There is thus a need for the development of novel senolytic drugs which can help prevent age-associated diseases more safely and effectively.
Previous studies have shown that caloric restriction or reduction of average daily caloric intake is associated with longevity and decreased tissue accumulation of senescent cells. In addition, inhibition of sodium glucose co-transporter 2 (SGLT2)—a key protein involved in glucose transport—is effective in reducing blood glucose levels leading to loss of calories. But can these effects be extended to senescent cell removal?
To find out, Professor Tohru Minamino and Dr. Goro Katsuumi from the Department of Cardiovascular Biology and Medicine, Juntendo University, Japan, along with their team of researchers, conducted a series of experiments to understand the potential senolytic effect of the SGLT2 inhibitor—canagliflozin, and its underlying molecular mechanisms. Giving further insight into their work published in Nature Aging on May 30, 2024, Prof. Minamino states, “Unlike the conventional senescent cell-eliminating agents, SGLT2 inhibitors are a new class of therapeutic agents with fewer side effects as they promote senescent cell elimination by activating the immune system.”
The researchers used a mouse model of dietary obesity, wherein mice were fed with a high fat diet (HFD) to induce cell senescence. Next, they treated the mice with canagliflozin (administered orally), and examined them for changes in glucose metabolism and HFD-induced senescence.
Notably, canagliflozin treatment led to significant improvement in glucose metabolism and reduced insulin resistance, compared to the HFD control animals. Further, the treatment led to a reduction in senescence-related markers including senescence-associated β-galactosidase (SA-β-gal) activity and pro-inflammatory senescence-associated secretary phenotype (SASP) factors. Furthermore, mice treated with canagliflozin showed decreased inflammation and oxidative stress in adipose tissues. Additionally, in vivo fluorescence studies also demonstrated that canagliflozin attenuated SA-β-gal activity and eliminated senescent cells.
Next, the researchers sought to uncover the molecular mechanisms underlying the senolytic activity of canagliflozin using metabolomic analysis (assessment of changes in metabolites). They found that SGLT2 inhibition led to a significant increase in the plasma level of 5-aminoimidazole-4-carboxamide 1-β-D-ribofuranoside (AICAR)—a metabolite known to activate adenosine monophosphate-activated protein kinase (AMPK). They also noted a subsequent increase in the levels of activated AMPK. Notably, mice treated with AICAR exhibited reduced SA-β-gal activity, similar to SGLT2 inhibition. Conversely, inhibition of AMPK led to an increase in SA-β-gal activity, corroborating the role of AICAR and AMPK in senolysis induced by SGLT2 inhibition.
The immune system and immune checkpoint factor – programmed cell death-1 (PD-1)/PD-L1 are known to be actively involved in cell senescence. Further, AMPK is known to negatively regulate the expression of PD-L1. Thus, the researchers speculated that AMPK activation following SGLT2 inhibition may regulate PD-L1 expression. Sure enough, SGLT2 inhibition with canagliflozin significantly reduced the previously elevated number of PD-L1-positive senescent cells in HFD-fed mice. Conversely, suppression of immune cells following canagliflozin treatment led to an increase in senescent cells, indicating that the senolytic effects of canagliflozin were partly mediated by the immune system.
In addition to the above findings, the researchers also noted that SGLT2 inhibition could ameliorate specific aging-induced pathologies by removing senescent cells, improving physical activity, and increasing the life span of treated animals. Overall, these findings highlight the clinical potential of SGLT2 inhibition as an effective senolytic strategy in the management of aging-related disorders.
Sharing his concluding thoughts, Prof. Minamino says, “In this study, we were able to demonstrate improvement against diabetes, arteriosclerosis, premature aging, and frailty, as well as a prolonged life span in response to SGLT2 inhibition. SGLT2 inhibitors may be applied in the treatment of various aging-related diseases, including Alzheimer’s disease, in the future.”
***
Reference
|
Authors |
Goro Katsuumi1, 2*, Ippei Shimizu3, 4*, Masayoshi Suda1*, Yohko Yoshida1, 5, Takaaki Furihata1, Yusuke Joki1, Chieh-Lun Hsiao1, Liang Jiaqi1, Shinya Fujiki2, Manabu Abe6, Masataka Sugimoto7, Tomoyoshi Soga8, 9, and Tohru Minamino1,10** |
|
Title of original paper |
SGLT2 inhibition eliminates senescent cells and alleviates pathological aging |
|
Journal |
Nature Aging |
|
DOI |
|
|
Affiliations |
1Department of Cardiovascular Biology and Medicine, Juntendo University Graduate, School of Medicine, Tokyo, Japan 2Department of Cardiovascular Medicine, Niigata University Graduate School of Medical and Dental Sciences, Niigata, Japan 3Department of Cardiovascular Aging, National Cerebral and Cardiovascular Center Research Institute, Osaka, Japan 4Department of Cardiovascular Medicine, National Cerebral and Cardiovascular Center, Osaka, Japan 5Department of Advanced Senotherapeutics, Juntendo University Graduate School of Medicine, Tokyo, Japan 6Department of Animal Model Development, Brain Research Institute, Niigata University, Niigata, Japan. 7Molecular and Cellular Aging, Tokyo Metropolitan Institute for Geriatrics and Gerontology, Tokyo, Japan 8Institute for Advanced Biosciences, Keio University, Yamagata, Japan 9Human Biology-Microbiome-Quantum Research Center (WPI-Bio2Q), Keio University, Tokyo, Japan 10Japan Agency for Medical Research and Development-Core Research for Evolutionary Medical Science and Technology (AMED-CREST), Japan Agency for Medical Research and Development, Tokyo, Japan |
***
About Professor Tohru Minamino
Professor Tohru Minamino is a Faculty and Chairman at the Department of Cardiovascular Biology and Medicine, Juntendo University Graduate School of Medicine, Japan. His lab focuses on the biology of aging, cardiovascular biology, regenerative medicine, metabolic disease, and cellular senescence. His research interests include elucidating the molecular mechanisms of age-associated disease (including cardiovascular and metabolic disease), the roles of cellular senescence in age-associated disease, the development of senolytic treatments, and regenerative therapy. He has authored 300 original articles and 45 review articles, which have been published in various peer-reviewed journals including Nature, Cell, and Nature Medicine.