Feb. 13, Thu, 2020
Topics
>RESEARCH
Light-activated protein treats Parkinson’s disease in a fly model
(Tokyo,13 February) A light-sensitive protein can restore mitochondria function in fruit flies bearing a gene mutation associated with Parkinson’s disease and relieve its symptoms, report researchers at Juntendo University in the journal Communications
Biology. The results provide a “proof of concept” for the approach as a potential treatment for Parkinson’s disease.
Parkinson’s disease is estimated to affect 10 million worldwide, with a prevalence of almost 2 in every 100 among those aged 80 and over. Functional failure in the mitochondria ? known as the power houses of cells ? is thought to lead to the disease’s many symptoms, which primarily impact mobility resulting in shaking, slowness and stiffness, but also include a number of physical, cognitive and psychological symptoms such as nerve pain, dizziness, dementia and depression. Yuzuru Imai and Nobutaka Hattori at Juntendo University and colleagues at the University of Shizuoka and Waseda University in Japan have now developed a light-activated protein, named mito-Delta-rhodopsin (mito-dR), that restores mitochondrion function in a fruit fly model of the disease.
Previous research has pointed towards mutations in the protein coding gene CHCHD2 as the cause of the disease, as well as identifying a number of cellular processes that the disease affects. In terms of energy generating processes, production of ATP – the energy currency in cells ? is diminished and the generation of reactive oxygen species increases. Other changes include an accumulation of α-synuclein, a protein mostly found at the tips of neurons, a lack of buffering of the levels of Ca2+ ions, which transfer signals around the nervous system, and ultimately a loss of dopaminergic neurons.
As a model to study the disease, Imai, Hattori and colleagues used Drosophila where the fly’s analogue for the CHCHD2 gene was made inoperative (knocked out). They found that decreased ATP production, increased reactive oxygen species, α-synuclein accumulation and loss of Ca2+-buffering, as well as neuron loss all resulted from the genetic modification.
Mitochondrial functions involve the interplay of complex reaction cycles, redox processes and electrochemical forces. In the hope of restoring the lost functions, the researchers examined the effects when the flies were treated with mitochondria designed to express a light-activated proton-transporter protein ? Delta-rhodopsin. They found that with exposure to light the mitochondria functions were restored in the flies expressing this protein in cell mitochondria. They also noted the beneficial effects on mitochondrial functions of dopaminergic terminals, increased dopamine production, locomotor activity and flight behavior.
The same functions were not restored in controls expressing mito-dR designed not to respond to light, confirming that the action of the light-activated protein and not the light itself was responsible for the observed recoveries. The researchers conclude that the results provide a ‘proof of concept’ for a potential therapeutic strategy for Parkinson’s disease.
Small amounts of energy released in the transfer of chemicals that feed into the electron transport chain can be used to pump protons into the intermembrane space, which establishes an electrochemical gradient across the inner membrane. Electrons that prematurely reduce oxygen in this process can lead to reactive oxygen species and cause oxidative stress to the mitochondria, which is related to age-related mitochondrial decline. Conversely proton leak can lower the production of reactive oxygen species and is thought to occur as mitochondrial proton-motive force rises.
Mitochondria can also store Ca2+ ions. Regulating the concentration of these ions affects signal reactions important for signal transduction in the cell. The release of these ions can lead to calcium ion spikes and changes in the membrane potential. It can also trigger the release of neurotransmitters in nerve cells and hormones in endocrine cells.
In their control experiments the researchers replace two of the key residues involved in the isomerization with non-functional amino acids. So that the control dR although present was not activated by light.
DOI: 10.1038/s42003-019-0674-1
URL: https://doi.org/10.1038/s42003-019-0674-1
Parkinson’s disease is estimated to affect 10 million worldwide, with a prevalence of almost 2 in every 100 among those aged 80 and over. Functional failure in the mitochondria ? known as the power houses of cells ? is thought to lead to the disease’s many symptoms, which primarily impact mobility resulting in shaking, slowness and stiffness, but also include a number of physical, cognitive and psychological symptoms such as nerve pain, dizziness, dementia and depression. Yuzuru Imai and Nobutaka Hattori at Juntendo University and colleagues at the University of Shizuoka and Waseda University in Japan have now developed a light-activated protein, named mito-Delta-rhodopsin (mito-dR), that restores mitochondrion function in a fruit fly model of the disease.
Previous research has pointed towards mutations in the protein coding gene CHCHD2 as the cause of the disease, as well as identifying a number of cellular processes that the disease affects. In terms of energy generating processes, production of ATP – the energy currency in cells ? is diminished and the generation of reactive oxygen species increases. Other changes include an accumulation of α-synuclein, a protein mostly found at the tips of neurons, a lack of buffering of the levels of Ca2+ ions, which transfer signals around the nervous system, and ultimately a loss of dopaminergic neurons.
As a model to study the disease, Imai, Hattori and colleagues used Drosophila where the fly’s analogue for the CHCHD2 gene was made inoperative (knocked out). They found that decreased ATP production, increased reactive oxygen species, α-synuclein accumulation and loss of Ca2+-buffering, as well as neuron loss all resulted from the genetic modification.
Mitochondrial functions involve the interplay of complex reaction cycles, redox processes and electrochemical forces. In the hope of restoring the lost functions, the researchers examined the effects when the flies were treated with mitochondria designed to express a light-activated proton-transporter protein ? Delta-rhodopsin. They found that with exposure to light the mitochondria functions were restored in the flies expressing this protein in cell mitochondria. They also noted the beneficial effects on mitochondrial functions of dopaminergic terminals, increased dopamine production, locomotor activity and flight behavior.
The same functions were not restored in controls expressing mito-dR designed not to respond to light, confirming that the action of the light-activated protein and not the light itself was responsible for the observed recoveries. The researchers conclude that the results provide a ‘proof of concept’ for a potential therapeutic strategy for Parkinson’s disease.

Fig.1
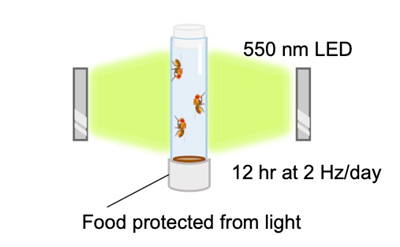
Diagram showing light stimulation of mitochondria in flies. Newly eclosed adult files were irradiated with 550 nm LED at 2 Hz for 12 hr every day. Fly food containing retinal, which was protected from light with aluminum foil, was changed with fresh food every day.
Diagram showing light stimulation of mitochondria in flies. Newly eclosed adult files were irradiated with 550 nm LED at 2 Hz for 12 hr every day. Fly food containing retinal, which was protected from light with aluminum foil, was changed with fresh food every day.

Fig.2
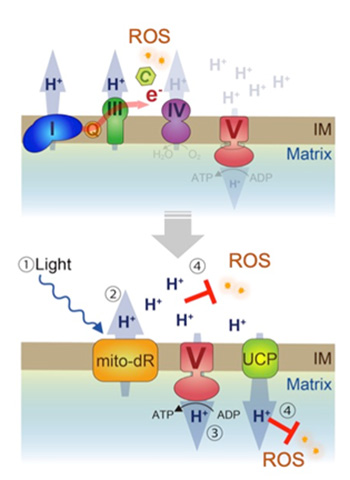
A model of mitochondrial rescue by mito-dR. (Upper) Loss of dCHCHD2 destabilizes cytochrome c, leading to OXPHOS dysfunction, decreased mitochondrial membrane potential, and reactive oxygen species (ROS) production. (Lower) Green light stimulates mitochondrion targeted mito-dR (1), which maintains mitochondrion proton-motive force through pumping out protons from the matrix to the intermembrane space (2), promoting proton influx-dependent ATP synthesis in complex V (3). Increased protons in the intermembrane space quench ROS in the intermembrane space directly and in the matrix by mild uncoupling via uncoupling proteins (4). IM, mitochondrial intermembrane. Courtesy Communications Biology.
A model of mitochondrial rescue by mito-dR. (Upper) Loss of dCHCHD2 destabilizes cytochrome c, leading to OXPHOS dysfunction, decreased mitochondrial membrane potential, and reactive oxygen species (ROS) production. (Lower) Green light stimulates mitochondrion targeted mito-dR (1), which maintains mitochondrion proton-motive force through pumping out protons from the matrix to the intermembrane space (2), promoting proton influx-dependent ATP synthesis in complex V (3). Increased protons in the intermembrane space quench ROS in the intermembrane space directly and in the matrix by mild uncoupling via uncoupling proteins (4). IM, mitochondrial intermembrane. Courtesy Communications Biology.
Researcher video(144 seconds)Yuzuru Imai, Senior associate professor,Department of Neurology, Juntendo University Faculty of Medicine.
Background
Redox reactions
Reactions that add oxygen atoms to a molecule are called oxidizing reactions and those that reduce the number of oxygen atoms are called reduction reactions. As an extension of their role in these reactions, the transfer of hydrogen ions (H+) and electrons are also described as oxidizing and reducing, or collectively redox, processes.Mitochondria and energy
Adenosine triphosphate (ATP) is the chemical responsible for capturing chemical energy from the breakdown of food so that it can be used to fuel the functions of cells throughout the body. Mitochondria produce ATP by oxidizing pyruvate and NADH, the main products of glucose. The process includes a series of reactions called the citric acid cycle. Pyruvate is actively transported across the inner mitochondrial membrane to the mitochondrial matrix where the citric cycle enzymes are found. The citric cycle also produces reduced cofactors that are a source of electrons in the electron transport train.Small amounts of energy released in the transfer of chemicals that feed into the electron transport chain can be used to pump protons into the intermembrane space, which establishes an electrochemical gradient across the inner membrane. Electrons that prematurely reduce oxygen in this process can lead to reactive oxygen species and cause oxidative stress to the mitochondria, which is related to age-related mitochondrial decline. Conversely proton leak can lower the production of reactive oxygen species and is thought to occur as mitochondrial proton-motive force rises.
Mitochondria can also store Ca2+ ions. Regulating the concentration of these ions affects signal reactions important for signal transduction in the cell. The release of these ions can lead to calcium ion spikes and changes in the membrane potential. It can also trigger the release of neurotransmitters in nerve cells and hormones in endocrine cells.
Delta-rhodopsin
Delta-rhodopsin (dR) is a type of bacteria that thrives in high salt concentrations. It drives unidirectional transmembrane ion transport in response to light through isomerization ? a rearrangement of the constituent atoms ? of a form of vitamin A known as retinal.In their control experiments the researchers replace two of the key residues involved in the isomerization with non-functional amino acids. So that the control dR although present was not activated by light.
Reference
Yuzuru Imai, Tsuyoshi Inoshita, Hongrui Meng, Kahori Shiba-Fukushima, Kiyotaka Y. Hara,Naoya Sawamura and Nobutaka Hattori. Light-driven activation of mitochondrial protonmotive force improves motor behaviors in a Drosophila model of Parkinson’s disease. Communications Biology, December 2019.DOI: 10.1038/s42003-019-0674-1
URL: https://doi.org/10.1038/s42003-019-0674-1